Who funded the research behind the Oxford-AstraZeneca COVID-19 vaccine?
- PMID: 34937701
- PMCID: PMC8704023
- DOI: 10.1136/bmjgh-2021-007321
Who funded the research behind the Oxford-AstraZeneca COVID-19 vaccine?
Abstract
Objectives: The Oxford-AstraZeneca COVID-19 vaccine (ChAdOx1 nCoV-19, Vaxzevira or Covishield) builds on two decades of research and development (R&D) into chimpanzee adenovirus-vectored vaccine (ChAdOx) technology at the University of Oxford. This study aimed to approximate the funding for the R&D of ChAdOx and the Oxford-AstraZeneca vaccine and to assess the transparency of funding reporting mechanisms.
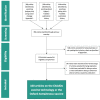
Methods: We conducted a scoping review and publication history analysis of the principal investigators to reconstruct R&D funding the ChAdOx technology. We matched award numbers with publicly accessible grant databases. We filed freedom of information (FOI) requests to the University of Oxford for the disclosure of all grants for ChAdOx R&D.
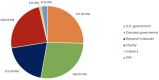
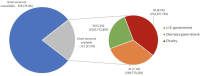
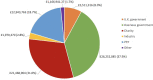
Results: We identified 100 peer-reviewed articles relevant to ChAdOx technology published between January 2002 and October 2020, extracting 577 mentions of funding bodies from acknowledgements. Government funders from overseas (including the European Union) were mentioned 158 times (27.4%), the UK government 147 (25.5%) and charitable funders 138 (23.9%). Grant award numbers were identified for 215 (37.3%) mentions; amounts were publicly available for 121 (21.0%). Based on the FOIs, until December 2019, the biggest funders of ChAdOx R&D were the European Commission (34.0%), Wellcome Trust (20.4%) and Coalition for Epidemic Preparedness Innovations (17.5%). Since January 2020, the UK government contributed 95.5% of funding identified. The total identified R&D funding was £104 226 076 reported in the FOIs and £228 466 771 reconstructed from the literature search.
Conclusion: Our study approximates that public and charitable financing accounted for 97%-99% of identifiable funding for the ChAdOx vaccine technology research at the University of Oxford underlying the Oxford-AstraZeneca vaccine until autumn 2020. We encountered a lack of transparency in research funding reporting.
Keywords: COVID-19; health policy; vaccines.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The authors of this paper are all members of Universities Allied for Essential Medicines Europe. SW is a member of the Executive Committee of Universities Allied for Essential Medicines Global and FR is the National Coordinator of Universities Allied for Essential Medicines UK. SK and RO are members of the People’s Health Movement and the WHO Watch initiative. RO is currently policy director for Students for Global Health UK. However, views expressed in this paper are their own and are not necessarily shared with the organisations the authors are affiliated with.
Figures

Similar articles
-
Sustained COVID-19 vaccine willingness after safety concerns over the Oxford-AstraZeneca vaccine.Dan Med J. 2021 Mar 31;68(5):A03210292. Dan Med J. 2021. PMID: 33870886
-
Efficacy of Oxford-AstraZeneca (ChAdOx1 CoV-19) vaccine against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) cases, hospital admissions, type of variants, and deaths.Eur Rev Med Pharmacol Sci. 2023 Oct;27(20):10133-10143. doi: 10.26355/eurrev_202310_34193. Eur Rev Med Pharmacol Sci. 2023. PMID: 37916383
-
Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial.EBioMedicine. 2022 Nov;85:104298. doi: 10.1016/j.ebiom.2022.104298. Epub 2022 Oct 10. EBioMedicine. 2022. PMID: 36229342 Free PMC article. Clinical Trial.
-
Perspectives on vaccine induced thrombotic thrombocytopenia.J Autoimmun. 2021 Jul;121:102663. doi: 10.1016/j.jaut.2021.102663. Epub 2021 May 18. J Autoimmun. 2021. PMID: 34020254 Free PMC article. Review.
-
MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature.J Neurol. 2022 Oct;269(10):5198-5212. doi: 10.1007/s00415-022-11194-9. Epub 2022 Jun 23. J Neurol. 2022. PMID: 35737110 Free PMC article. Review.
Cited by
-
Wars and sweets: microbes, medicines and other moderns in and beyond the(ir) antibiotic era.Med Humanit. 2022 Aug 10;48(3):359-70. doi: 10.1136/medhum-2021-012366. Online ahead of print. Med Humanit. 2022. PMID: 35948395 Free PMC article.
-
Global COVID-19 Vaccine Inequity: Failures in the First Year of Distribution and Potential Solutions for the Future.Front Public Health. 2022 Mar 7;10:821117. doi: 10.3389/fpubh.2022.821117. eCollection 2022. Front Public Health. 2022. PMID: 35321196 Free PMC article.
-
Which roads lead to access? A global landscape of six COVID-19 vaccine innovation models.Global Health. 2024 Mar 26;20(1):25. doi: 10.1186/s12992-024-01017-z. Global Health. 2024. PMID: 38532484 Free PMC article.
-
Shedding Lights on the Extracellular Vesicles as Functional Mediator and Therapeutic Decoy for COVID-19.Life (Basel). 2023 Mar 20;13(3):840. doi: 10.3390/life13030840. Life (Basel). 2023. PMID: 36983995 Free PMC article. Review.
-
COVID-19 vaccine apartheid and the failure of global cooperation.Br J Polit Int Relat. 2023 Aug;25(3):535-554. doi: 10.1177/13691481231178248. Epub 2023 Jun 13. Br J Polit Int Relat. 2023. PMID: 38602976 Free PMC article.
References
-
- Medicines & Healthcare products Regulatory Agency . Conditions of authorisation for COVID-19 vaccine AstraZeneca, 2021. Available: https://www.gov.uk/government/publications/regulatory-approval-of-covid-... [Accessed 29 Mar 2021].
-
- UK Government . Approved COVID-19 vaccines and countries and territories with approved proof of vaccination. Available: https://www.gov.uk/guidance/countries-with-approved-covid-19-vaccination... [Accessed 1 Nov 2021].
-
- University of Oxford . Oxford vaccine reaches one billion doses released, 2021. Available: https://www.ox.ac.uk/news/2021-07-29-oxford-vaccine-reaches-one-billion-... [Accessed 1 Nov 2021].
-
- Ramasamy MN, Minassian AM, Ewer KJ, et al. . Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021;396:1979–93. 10.1016/S0140-6736(20)32466-1 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
