Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis
- PMID: 34937766
- PMCID: PMC9484392
- DOI: 10.1136/gutjnl-2020-323951
Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis
Abstract
Objective: Using faecal shotgun metagenomic sequencing, we identified the depletion of Lactobacillus gallinarum in patients with colorectal cancer (CRC). We aimed to determine the potential antitumourigenic role of L. gallinarum in colorectal tumourigenesis.
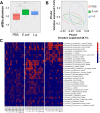
Design: The tumor-suppressive effect of L. gallinarum was assessed in murine models of CRC. CRC cell lines and organoids derived from patients with CRC were cultured with L. gallinarum or Escherichia coli MG1655 culture-supernatant to evaluate cell proliferation, apoptosis and cell cycle distribution. Gut microbiota was assessed by 16S ribosomal DNA sequencing. Antitumour molecule produced from L. gallinarum was identified by liquid chromatography mass spectrometry (LC-MS/MS) and targeted mass spectrometry.
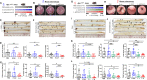
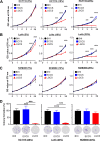
Results: L. gallinarum significantly reduced intestinal tumour number and size compared with E. coli MG1655 and phosphate-buffered saline in both male and female murine intestinal tumourigenesis models. Faecal microbial profiling revealed enrichment of probiotics and depletion of pathogenic bacteria in L. gallinarum-treated mice. Culturing CRC cells with L. gallinarum culture-supernatant (5%, 10% and 20%) concentration-dependently suppressed cell proliferation and colony formation. L. gallinarum culture-supernatant significantly promoted apoptosis in CRC cells and patient-derived CRC organoids, but not in normal colon epithelial cells. Only L. gallinarum culture-supernatant with fraction size <3 kDa suppressed proliferation in CRC cells. Using LC-MS/MS, enrichments of indole-3-lactic acid (ILA) was identified in both L. gallinarum culture-supernatant and the gut of L. gallinarum-treated mice. ILA displayed anti-CRC growth in vitro and inhibited intestinal tumourigenesis in vivo.
Conclusion: L. gallinarum protects against intestinal tumourigenesis by producing protective metabolites that can promote apoptosis of CRC cells.
Keywords: colorectal cancer; probiotics.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
