Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2
- PMID: 34937855
- PMCID: PMC8695582
- DOI: 10.1038/s41598-021-03461-y
Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2
Abstract
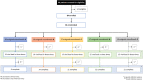
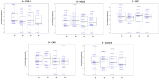
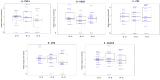
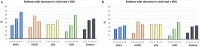
Most public health measures to contain the COVID-19 pandemic are based on preventing the pathogen spread, and the use of oral antiseptics has been proposed as a strategy to reduce transmission risk. The aim of this manuscript is to test the efficacy of mouthwashes to reduce salivary viral load in vivo. This is a multi-centre, blinded, parallel-group, placebo-controlled randomised clinical trial that tests the effect of four mouthwashes (cetylpyridinium chloride, chlorhexidine, povidone-iodine and hydrogen peroxide) in SARS-CoV-2 salivary load measured by qPCR at baseline and 30, 60 and 120 min after the mouthrinse. A fifth group of patients used distilled water mouthrinse as a control. Eighty-four participants were recruited and divided into 12-15 per group. There were no statistically significant changes in salivary viral load after the use of the different mouthwashes. Although oral antiseptics have shown virucidal effects in vitro, our data show that salivary viral load in COVID-19 patients was not affected by the tested treatments. This could reflect that those mouthwashes are not effective in vivo, or that viral particles are not infective but viral RNA is still detected by PCR. Viral infectivity studies after the use of mouthwashes are therefore required. ( https://clinicaltrials.gov/ct2/show/NCT04707742 ; Identifier: NCT04707742).
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

