Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma
- PMID: 34937871
- PMCID: PMC8695578
- DOI: 10.1038/s41467-021-26472-9
Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma
Abstract
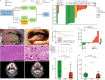
Surgery for locoregionally advanced head and neck squamous cell carcinoma (HNSCC) results in 30‒50% five-year overall survival. In IMCISION (NCT03003637), a non-randomized phase Ib/IIa trial, 32 HNSCC patients are treated with 2 doses (in weeks 1 and 3) of immune checkpoint blockade (ICB) using nivolumab (NIVO MONO, n = 6, phase Ib arm A) or nivolumab plus a single dose of ipilimumab (COMBO, n = 26, 6 in phase Ib arm B, and 20 in phase IIa) prior to surgery. Primary endpoints are feasibility to resect no later than week 6 (phase Ib) and primary tumor pathological response (phase IIa). Surgery is not delayed or suspended for any patient in phase Ib, meeting the primary endpoint. Grade 3‒4 immune-related adverse events are seen in 2 of 6 (33%) NIVO MONO and 10 of 26 (38%) total COMBO patients. Pathological response, defined as the %-change in primary tumor viable tumor cell percentage from baseline biopsy to on-treatment resection, is evaluable in 17/20 phase IIa patients and 29/32 total trial patients (6/6 NIVO MONO, 23/26 COMBO). We observe a major pathological response (MPR, 90‒100% response) in 35% of patients after COMBO ICB, both in phase IIa (6/17) and in the whole trial (8/23), meeting the phase IIa primary endpoint threshold of 10%. NIVO MONO's MPR rate is 17% (1/6). None of the MPR patients develop recurrent HSNCC during 24.0 months median postsurgical follow-up. FDG-PET-based total lesion glycolysis identifies MPR patients prior to surgery. A baseline AID/APOBEC-associated mutational profile and an on-treatment decrease in hypoxia RNA signature are observed in MPR patients. Our data indicate that neoadjuvant COMBO ICB is feasible and encouragingly efficacious in HNSCC.
© 2021. The Author(s).
Conflict of interest statement
J.L.V., J.B.W.E., J.J.H.T., X.Q., A.vd.L., Y.L., I.S., L.S., S.O., B.J., W.V.V., A.A.M., V.vd.N., A.K., E.H., A.B., R.D., L.K., M.B.K., P.J.F.M.L., W.H.S., W.M.C.K., L.vd.V., and I.B.T. declare no competing interests. C.L.Z. reports receiving institutional research financial support from BMS to fund the present trial. M.W.M.vd.B. reports, outside the submitted work, institutional research funding from ATOS Medical. S.M.W. reports, all outside the submitted work: institutional research funding from Roche, Pfizer, MSD, Bayer, Amgen, BMS, AstraZeneca, Lilly and Nextcure. O.K. is employed at Neogene Therapeutics B.V., though at time of analysis and writing his employment was at the NKI. O.K. further reports, outside the submitted work, a pending patent application (title: “Gene signatures and method for predicting response to pd-1 antagonists and ctla-4 antagonists, and combination thereof”, number: WO2020005068A8). D.S.P. reports, all outside the submitted work, a pending patent application (title: “Gene signatures and method for predicting response to pd-1 antagonists and ctla-4 antagonists, and combination thereof”, number: WO2020005068A8) and a role as co-founder, shareholder and advisor of Immagene. J.P.d.B. reports, all outside the submitted work: institutional research funding from Merck KGaA; institutional honoraria for an advisory role for MSD. T.N.M.S. reports, all outside the submitted work: advisory roles for Adaptive Biotechnologies, AIMM Therapeutics, Allogene Therapeutics, Merus, Neogene Therapeutics, Neon Therapeutics and Scenic Biotech; research support from Merck KGaA; stockholdership of AIMM Therapeutics, Allogene Therapeutics, Merus, BioNTech, Neogene Therapeutics, and Scenic Biotech. C.U.B. reports, all outside the submitted work: institutional research funding from BMS, Novartis and Nanostring; institutional honoraria for advisory roles for BMS, MSD, Roche, Novartis, GSK, AZ, Pfizer, Lilly, Genmab and Pierre Fabre; personal honoraria for an advisory role for Third Rock Ventures; stock ownership of Uniti Cars and Immagene. J.B.A.G.H. reports, all outside the submitted work: institutional honoraria for advisory roles for AIMM, Amgen, BioNTech, BMS, GSK, Ipsen, MSD, Merck Serono, Molecular Partners, Neogene Therapeutics, Novartis, Pfizer, Roche/Genentech, Sanofi, Seattle Genetics, Third Rock Ventures, Vaximm; stock option ownership of Neogene Therapeutics; Institutional research funding from Amgen, BioNTech, BMS, MSD, Novartis.
Figures

References
-
- Gatta G, et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer. 2015;51:2130–2143. - PubMed
-
- Bernier J, et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2004;350:1945–1952. - PubMed
-
- Cooper JS, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004;350:1937–1944. - PubMed
-
- Licitra L, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 2006;24:5630–5636. - PubMed
-
- Goodwin WJ. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110:1–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

