Rapid microbial methanogenesis during CO2 storage in hydrocarbon reservoirs
- PMID: 34937895
- PMCID: PMC8695373
- DOI: 10.1038/s41586-021-04153-3
Rapid microbial methanogenesis during CO2 storage in hydrocarbon reservoirs
Abstract
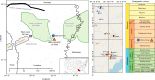
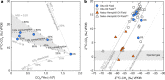
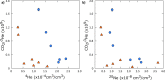
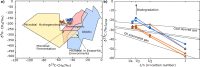
Carbon capture and storage (CCS) is a key technology to mitigate the environmental impact of carbon dioxide (CO2) emissions. An understanding of the potential trapping and storage mechanisms is required to provide confidence in safe and secure CO2 geological sequestration1,2. Depleted hydrocarbon reservoirs have substantial CO2 storage potential1,3, and numerous hydrocarbon reservoirs have undergone CO2 injection as a means of enhanced oil recovery (CO2-EOR), providing an opportunity to evaluate the (bio)geochemical behaviour of injected carbon. Here we present noble gas, stable isotope, clumped isotope and gene-sequencing analyses from a CO2-EOR project in the Olla Field (Louisiana, USA). We show that microbial methanogenesis converted as much as 13-19% of the injected CO2 to methane (CH4) and up to an additional 74% of CO2 was dissolved in the groundwater. We calculate an in situ microbial methanogenesis rate from within a natural system of 73-109 millimoles of CH4 per cubic metre (standard temperature and pressure) per year for the Olla Field. Similar geochemical trends in both injected and natural CO2 fields suggest that microbial methanogenesis may be an important subsurface sink of CO2 globally. For CO2 sequestration sites within the environmental window for microbial methanogenesis, conversion to CH4 should be considered in site selection.
© 2021. The Author(s).
Conflict of interest statement
M.F. and B.S. are employed by/an employee of ExxonMobil Upstream Integrated Solutions Company. Z.M.S. is employed by/an employee of ExxonMobil Research and Engineering Company. When the manuscript was first submitted, M.L. was employed by/an employee of ExxonMobil Upstream Integrated Solutions Company. M.L. is now employed by/an employee of Aker BP. The views expressed are those of the author(s) and not necessarily those of ExxonMobil Upstream Integrated Solutions Company, ExxonMobil Research and Engineering Company, or Aker BP.
Figures






References
-
- Leung DYC, Caramanna G, Maroto-Valer MM. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014;39:426–443.
-
- Bui M, et al. Carbon capture and storage (CCS): the way forward. Energy Environ. Sci. 2018;11:1062–1176.
-
- Stewart, R. J. & Haszeldine, R. S. Carbon Accounting for Carbon Dioxide Enhanced Oil Recovery (Scottish Carbon Capture & Storage, 2014); https://www.sccs.org.uk/images/expertise/misc/SCCS-CO2-EOR-JIP-Carbon-Ba...
-
- Gilfillan SMV, et al. Solubility trapping in formation water as dominant CO2 sink in natural gas fields. Nature. 2009;458:614–618. - PubMed
-
- Matter JM, et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science. 2016;352:1312–1314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

