NFAT inhibitor 11R-VIVIT ameliorates mouse renal fibrosis after ischemia-reperfusion-induced acute kidney injury
- PMID: 34937917
- PMCID: PMC9343462
- DOI: 10.1038/s41401-021-00833-y
NFAT inhibitor 11R-VIVIT ameliorates mouse renal fibrosis after ischemia-reperfusion-induced acute kidney injury
Abstract
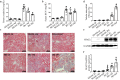
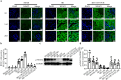
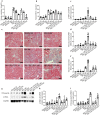
Acute kidney injury (AKI) with maladaptive tubular repair leads to renal fibrosis and progresses to chronic kidney disease (CKD). At present, there is no curative drug to interrupt AKI-to-CKD progression. The nuclear factor of the activated T cell (NFAT) family was initially identified as a transcription factor expressed in most immune cells and involved in the transcription of cytokine genes and other genes critical for the immune response. NFAT2 is also expressed in renal tubular epithelial cells (RTECs) and podocytes and plays an important regulatory role in the kidney. In this study, we investigated the renoprotective effect of 11R-VIVIT, a peptide inhibitor of NFAT, on renal fibrosis in the AKI-to-CKD transition and the underlying mechanisms. We first examined human renal biopsy tissues and found that the expression of NFAT2 was significantly increased in RTECs in patients with severe renal fibrosis. We then established a mouse model of AKI-to-CKD transition using bilateral ischemia-reperfusion injury (Bi-IRI). The mice were treated with 11R-VIVIT (5 mg/kg, i.p.) on Days 1, 3, 10, 17 and 24 after Bi-IRI. We showed that the expression of NFAT2 was markedly increased in RTECs in the AKI-to-CKD transition. 11R-VIVIT administration significantly inhibited the nuclear translocation of NFAT2 in RTECs, decreased the levels of serum creatinine and blood urea nitrogen, and attenuated renal tubulointerstitial fibrosis but had no toxic side effects on the heart and liver. In addition, we showed that 11R-VIVIT administration alleviated RTEC apoptosis after Bi-IRI. Consistently, preapplication of 11R-VIVIT (100 nM) and transfection with NFAT2-targeted siRNA markedly suppressed TGFβ-induced HK-2 cell apoptosis in vitro. In conclusion, 11R-VIVIT administration inhibits IRI-induced NFAT2 activation and prevents AKI-to-CKD progression. Inhibiting NFAT2 may be a promising new therapeutic strategy for preventing renal fibrosis after IR-AKI.
Keywords: 11R-VIVIT; HK-2 cells; NFAT2; acute kidney injury; apoptosis; chronic kidney disease; renal fibrosis; renal tubular epithelial cells.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95:160–72. doi: 10.1016/j.kint.2018.08.036. - DOI - PubMed
-
- Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;7:743–57. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

