Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection
- PMID: 34937928
- PMCID: PMC8709786
- DOI: 10.1038/s41590-021-01089-8
Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection
Abstract
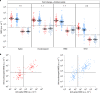
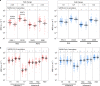
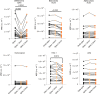
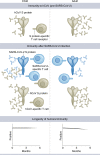
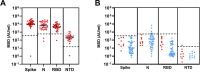
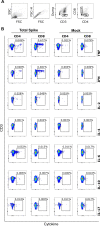
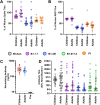
SARS-CoV-2 infection is generally mild or asymptomatic in children but a biological basis for this outcome is unclear. Here we compare antibody and cellular immunity in children (aged 3-11 years) and adults. Antibody responses against spike protein were high in children and seroconversion boosted responses against seasonal Beta-coronaviruses through cross-recognition of the S2 domain. Neutralization of viral variants was comparable between children and adults. Spike-specific T cell responses were more than twice as high in children and were also detected in many seronegative children, indicating pre-existing cross-reactive responses to seasonal coronaviruses. Importantly, children retained antibody and cellular responses 6 months after infection, whereas relative waning occurred in adults. Spike-specific responses were also broadly stable beyond 12 months. Therefore, children generate robust, cross-reactive and sustained immune responses to SARS-CoV-2 with focused specificity for the spike protein. These findings provide insight into the relative clinical protection that occurs in most children and might help to guide the design of pediatric vaccination regimens.
© 2021. The Author(s).
Conflict of interest statement
M.E.R. provided post-marketing surveillance reports on pneumococcal and meningococcal infection to vaccine manufacturers for which a cost recovery charge was made to GSK and Pfizer. The other authors declare no competing interests.
Figures



Comment in
-
Understanding Kids and COVID.Proc Natl Acad Sci U S A. 2022 Mar 29;119(13):e2203753119. doi: 10.1073/pnas.2203753119. Epub 2022 Mar 22. Proc Natl Acad Sci U S A. 2022. PMID: 35316141 Free PMC article. No abstract available.
-
Why are children less affected than adults by severe acute respiratory syndrome coronavirus 2 infection?Cell Mol Immunol. 2022 May;19(5):555-557. doi: 10.1038/s41423-022-00857-2. Epub 2022 Mar 24. Cell Mol Immunol. 2022. PMID: 35332299 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

