Rapid Emergence of T Follicular Helper and Germinal Center B Cells Following Antiretroviral Therapy in Advanced HIV Disease
- PMID: 34938286
- PMCID: PMC8686113
- DOI: 10.3389/fimmu.2021.752782
Rapid Emergence of T Follicular Helper and Germinal Center B Cells Following Antiretroviral Therapy in Advanced HIV Disease
Abstract
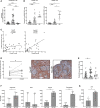
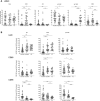
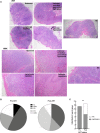
Low nadir CD4 T-cell counts in HIV+ patients are associated with high morbidity and mortality and lasting immune dysfunction, even after antiretroviral therapy (ART). The early events of immune recovery of T cells and B cells in severely lymphopenic HIV+ patients have not been fully characterized. In a cohort of lymphopenic (CD4 T-cell count < 100/µL) HIV+ patients, we studied mononuclear cells isolated from peripheral blood (PB) and lymph nodes (LN) pre-ART (n = 40) and 6-8 weeks post-ART (n = 30) with evaluation of cellular immunophenotypes; histology on LN sections; functionality of circulating T follicular helper (cTfh) cells; transcriptional and B-cell receptor profile on unfractionated LN and PB samples; and plasma biomarker measurements. A group of 19 healthy controls (HC, n = 19) was used as a comparator. T-cell and B-cell lymphopenia was present in PB pre-ART in HIV+ patients. CD4:CD8 and CD4 T- and B-cell PB subsets partly normalized compared to HC post-ART as viral load decreased. Strikingly in LN, ART led to a rapid decrease in interferon signaling pathways and an increase in Tfh, germinal center and IgD-CD27- B cells, consistent with histological findings of post-ART follicular hyperplasia. However, there was evidence of cTfh cells with decreased helper capacity and of limited B-cell receptor diversification post-ART. In conclusion, we found early signs of immune reconstitution, evidenced by a surge in LN germinal center cells, albeit limited in functionality, in HIV+ patients who initiate ART late in disease.
Keywords: HIV; T follicular helper cells; germinal centers; interferon; reconstitution.
Copyright © 2021 Wong, Buckner, Lage, Pei, Assis, Dahlstrom, Anzick, Virtaneva, Rupert, Davis, Zhou, Laidlaw, Manion, Galindo, Anderson, Seamon, Sneller, Lisco, Deleage, Pittaluga, Moir and Sereti.
Conflict of interest statement
Author AR was employed by company Leidos Biomedical Research Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Boulle A, Schomaker M, May MT, Hogg RS, Shepherd BE, Monge S, et al. . Mortality in Patients With HIV-1 Infection Starting Antiretroviral Therapy in South Africa, Europe, or North America: A Collaborative Analysis of Prospective Studies. PloS Med (2014) 11(9):e1001718. doi: 10.1371/journal.pmed.1001718 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

