The Impact of Age Difference on the Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis
- PMID: 34938287
- PMCID: PMC8685545
- DOI: 10.3389/fimmu.2021.758294
The Impact of Age Difference on the Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis
Abstract
Objective: This meta-analysis compared the efficacy and safety of five kinds of COVID-19 vaccines in different age groups (young adults and older adults), aiming to analyze the difference of adverse events (AEs) rate and virus geometric mean titer (GMT) values between young and older people, in order to find a specific trend, and explore the causes of this trend through meta-analysis.
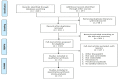
Method: Meta-analysis was used to analyze the five eligible articles. The modified Jadad scoring scale was used to evaluate the quality of eligible literature with a scoring system of 1 to 7. The primary endpoint of the effectiveness index was GMT. The primary endpoints of the safety index were the incidence of local AEs and systemic AEs. Stata 12.0 software was used for meta-analysis. Revman 5.0 software was used to map the risk of publication bias, and Egger's test was used to analyze publication bias.
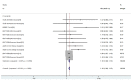
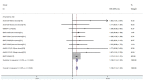
Results: The GMT values of young adults were higher than older adults (SMD = 1.40, 95% CI (0.79, 2.02), P<0.01). There was a higher incidence of local and systemic AEs in young people than in the elderly (OR = 1.10, 95% CI (1.08, 1.12), P<0.01; OR = 1.18, 95% CI (1.14, 1.22), P<0.01).
Conclusion: The immune effect of young people after being vaccinated with COVID-19 vaccines was better than that of the elderly, but the safety was worse than that of old people, the most common AEs were fever, rash, and local muscle pain, which were tolerable for young people. As the AEs of the elderly were lower, they can also be vaccinated safely; the reason for the low level of GMT in the elderly was related to Immunosenescence. The vaccine tolerance of people of different ages needs to be studied continuously.
Keywords: COVID-19 vaccines; age; double-blind; efficacy and safety; meta-analysis; randomized-controlled trials (RCT).
Copyright © 2021 Wang, Tong, Li, Li and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- World Health Organization . WHO Coronavirus Disease (COVID-19) Dashboard [EB/OL](2021-06-28). Available at: https://covid19.who.int/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

