DdCBE mediates efficient and inheritable modifications in mouse mitochondrial genome
- PMID: 34938607
- PMCID: PMC8646052
- DOI: 10.1016/j.omtn.2021.11.016
DdCBE mediates efficient and inheritable modifications in mouse mitochondrial genome
Abstract
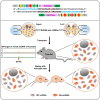
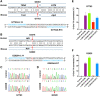
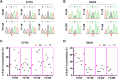
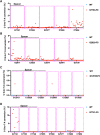
Critical mutations of mitochondrial DNA (mtDNA) generally lead to maternally inheritable diseases that affect multiple organs and systems; however, it was difficult to alter mtDNA in mammalian cells to intervene in or cure mitochondrial disorders. Recently, the discovery of DddA-derived cytosine base editor (DdCBE) enabled the precise manipulation of mtDNA. To test its feasibility for in vivo use, we selected several sites in mouse mtDNA as DdCBE targets to resemble the human pathogenic mtDNA G-to-A mutations. The efficiency of DdCBE-mediated mtDNA editing was first screened in mouse Neuro-2A cells and DdCBE pairs with the best performance were chosen for in vivo targeting. Microinjection of the mRNAs of DdCBE halves in the mouse zygotes or 2-cell embryo successfully generated edited founder mice with a base conversion rate ranging from 2.48% to 28.51%. When backcrossed with wild-type male mice, female founders were able to transmit the mutations to their offspring with different mutation loads. Off-target analyses demonstrated a high fidelity for DdCBE-mediated base editing in mouse mtDNA both in vitro and in vivo. Our study demonstrated that the DdCBE is feasible for generation of mtDNA mutation models to facilitate disease study and for potential treatment of mitochondrial disorders.
Keywords: DdCBE; base editing; mitochondrial disorder; mouse model; mtDNA.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 1985;54:1015–1069. - PubMed
-
- Pozzan T., Magalhaes P., Rizzuto R. The comeback of mitochondria to calcium signalling. Cell Calcium. 2000;28:279–283. - PubMed
-
- Newmeyer D.D., Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. - PubMed
-
- Scheffler I.E. Mitochondria. Second edition. John Wiley & Sons, 2007. Metabolic pathways inside mitochondria; pp. 298–344.
LinkOut - more resources
Full Text Sources

