How microbial glycosyl hydrolase activity in the gut mucosa initiates microbial cross-feeding
- PMID: 34939101
- PMCID: PMC8966484
- DOI: 10.1093/glycob/cwab105
How microbial glycosyl hydrolase activity in the gut mucosa initiates microbial cross-feeding
Abstract
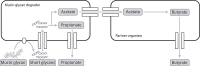
The intestinal epithelium is protected from direct contact with gut microbes by a mucus layer. This mucus layer consists of secreted mucin glycoproteins. The outer mucus layer in the large intestine forms a niche that attracts specific gut microbiota members of which several gut commensals can degrade mucin. Mucin glycan degradation is a complex process that requires a broad range of glycan degrading enzymes, as mucin glycans are intricate and diverse molecules. Consequently, it is hypothesized that microbial mucin breakdown requires concerted action of various enzymes in a network of multiple resident microbes in the gut mucosa. This review investigates the evolutionary relationships of microbial carbohydrate-active enzymes that are potentially involved in mucin glycan degradation and focuses on the role that microbial enzymes play in the degradation of gut mucin glycans in microbial cross-feeding and syntrophic interactions.
Keywords: CAZymes; glycosidases; gut microbiota; mucin; syntrophic interactions.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

