Multimodal Enzyme-Carrying Suprastructures for Rapid and Sensitive Biocatalytic Cascade Reactions
- PMID: 34939366
- PMCID: PMC8981434
- DOI: 10.1002/advs.202104884
Multimodal Enzyme-Carrying Suprastructures for Rapid and Sensitive Biocatalytic Cascade Reactions
Erratum in
-
Multimodal Enzyme-Carrying Suprastructures for Rapid and Sensitive Biocatalytic Cascade Reactions.Adv Sci (Weinh). 2022 Sep;9(27):e2204665. doi: 10.1002/advs.202204665. Adv Sci (Weinh). 2022. PMID: 36150221 Free PMC article. No abstract available.
Abstract
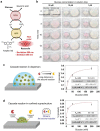
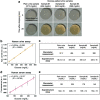
Colloidal assemblies of mesoporous suprastructures provide effective catalysis in an advantageous volume-confined environment. However, typical fabrication methods of colloidal suprastructures are carried out under toxic or harmful conditions for unstable biomolecules, such as, biocatalytic enzymes. For this reason, biocatalytic enzymes have rarely been used with suprastructures, even though biocatalytic cascade reactions in confined environments are more efficient than in open conditions. Here, multimodal enzyme- and photocatalyst-carrying superstructures with efficient cascade reactions for colorimetric glucose detection are demonstrated. The suprastructures consisting of various functional nanoparticles, including enzyme-carrying nanoparticles, are fabricated by surface-templated evaporation driven suprastructure synthesis on polydimethylsiloxane-grafted surfaces at ambient conditions. For the fabrication of suprastructures, no additional chemicals and reactions are required, which allows maintaining the enzyme activities. The multimodal enzymes (glucose oxidase and peroxidase)-carrying suprastructures exhibit rapid and highly sensitive glucose detection via two enzyme cascade reactions in confined geometry. Moreover, the combination of enzymatic and photocatalytic cascade reactions of glucose oxidase to titanium dioxide nanoparticles is successfully realized for the same assay. These results show promising abilities of multiple colloidal mixtures carrying suprastructures for effective enzymatic reactions and open a new door for advanced biological reactions and enzyme-related works.
Keywords: biocatalytic cascade reactions; colloidal suprastructures; glucose assays; liquid repellent surfaces; surface-templated suprastructure synthesis.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yang S.‐M., Kim S.‐H., Lim J.‐M., Yi G.‐R., J. Mater. Chem. 2008, 18, 2177.
-
- Wintzheimer S., Granath T., Oppmann M., Kister T., Thai T., Kraus T., Vogel N., Mandel K., ACS Nano 2018, 12, 5093. - PubMed
-
- Piccinini E., Pallarola D., Battaglini F., Azzaroni O., Mol. Syst. Des. Eng. 2016, 1, 155.
-
- Yu Z., Wang C. F., Ling L., Chen L., Chen S., Angew. Chem., Int. Ed. 2012, 124, 2425.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
