SARS-CoV-2 infection: efficacy of extensive vaccination of the healthcare workforce in a large Italian hospital
- PMID: 34939622
- PMCID: PMC8759049
- DOI: 10.23749/mdl.v112i6.12124
SARS-CoV-2 infection: efficacy of extensive vaccination of the healthcare workforce in a large Italian hospital
Abstract
Background: A prospective observational study involved 13,787 Health Care Workers (HCWs) of a large hospital to assess the effectiveness of a SARS-CoV-2 mRNA vaccine.
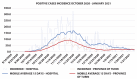
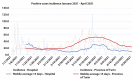
Methods: The daily incidence of infections was estimated from 1st October 2020 to 30th April 2021 and compared with that of the province of Turin (2.26 million). In the middle of this period, a mass vaccination began among HCW, and its effect was assessed.
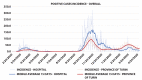
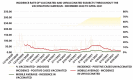
Results: In the first half-period, 1,163 positive HCWs were observed, the average daily incidence rate per 100,000 being 79.58 (± 15.58; 95% CI) compared to 38.54 (± 5.96; 95% CI) in the general population (p<0.001). The vaccination campaign immunized 9,843 HCWs; among them, the average daily incidence was 14.23 (± 2.73; 95% CI) compared to 34.2 (± 2.95; 95% CI) in the province (p<0.001). Among fully vaccinated HCW, 59 cases were observed, giving rise to an incidence of 6.3 (± 2.66; 95% CI) much lower than in the province (p<0.001). In the second half of the observation period, the RR for HCWs compared to the province dropped from 2.07 (1.96 - 2.18; 95% CI; p<0.001) to 0.5 (0.42 - 0.58; 95% CI; p<0.001) and to 0.17 (0.13 - 0.22; 95% CI; p<0.001) for unvaccinated and vaccinated HCWs, respectively. The RR of vaccinate HCW was 0.43 (0.31 - 0.58; 95% CI; p<0.001) compared to unvaccinated. In the second half of the observation period, unvaccinated HCWs had a RR of 0.21 (0.18 - 0.25; 95% CI; p<0.001) as compared to the first one. A linear regression model (R2 = 0.87) showed that every percent increase in vaccinated HCWs lowered daily incidence by 0.94 (0.86 - 1.02; IC 95%; p<0.001). Vaccinated HCWs had a RR of 0.09 (0.07 - 0.12; 95% CI; p<0.001) compared to unvaccinated HCWs, which led to estimated effectiveness of the two-dose vaccine of 91 % (± 3 %; CI 95%) similar to that reported by the manufacturer.
Figures

References
-
- World Health Organization. Coronavirus Disease (COVID-19) Situation Dashboard. 2021. Accessed June 10, 2021. https://covid19.who.int/
-
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 — 11 March 2020. 2020 Accessed June 10, 2021. https://www.who.int/director-general/speeches/detail/who-director-genera... .
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2021. 2021. Accessed June 10, 2021. https://covid19.who.int/
-
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi:10.1038/s41586-020-2798-3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

