Changes in Brain Energy and Membrane Metabolism in Glioblastoma following Chemoradiation
- PMID: 34940063
- PMCID: PMC8700426
- DOI: 10.3390/curroncol28060424
Changes in Brain Energy and Membrane Metabolism in Glioblastoma following Chemoradiation
Abstract
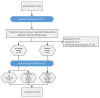
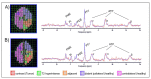
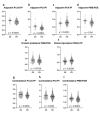
Brain parenchyma infiltration with glioblastoma (GB) cannot be entirely visualized by conventional magnetic resonance imaging (MRI). The aim of this study was to investigate changes in the energy and membrane metabolism measured with phosphorous MR spectroscopy (31P-MRS) in the presumably "normal-appearing" brain following chemoradiation therapy (CRT) in GB patients in comparison to healthy controls. Twenty (seven female, thirteen male) GB patients underwent a 31P-MRS scan prior to surgery (baseline) and after three months of standard CRT (follow-up examination. The regions of interest "contrast-enhancing (CE) tumor" (if present), "adjacent to the (former) tumor", "ipsilateral distant" hemisphere, and "contralateral" hemisphere were compared, differentiating between patients with stable (SD) and progressive disease (PD). Metabolite ratios PCr/ATP, Pi/ATP, PCr/Pi, PME/PDE, PME/PCr, and PDE/ATP were investigated. In PD, energy and membrane metabolism in CE tumor areas have a tendency to "normalize" under therapy. In different "normal-appearing" brain areas of GB patients, the energy and membrane metabolism either "normalized" or were "disturbed", in comparison to baseline or controls. Differences were also detected between patients with SD and PD. 31P-MRS might contribute as an additional imaging biomarker for outcome measurement, which remains to be investigated in a larger cohort.
Keywords: ATP; cerebral energy metabolism; chemoradiation; glioblastoma; normal-appearing brain tissue; phosphorous magnetic resonance spectroscopy (31P-MRS); tumor infiltration.
Conflict of interest statement
The authors have no conflict of interests to declare. The funders played no role in the design of the study; collection, analyses, or interpretation of data; the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Scherer H.J. Structural development in gliomas. Am. J. Cancer. 1938;34:333–351.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

