Durable Response to Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, and Prednisone (BV-CHP) in a Patient with CD30-Positive PTCL Arising as a Post-Transplant Lymphoproliferative Disorder (PTLD)
- PMID: 34940065
- PMCID: PMC8699839
- DOI: 10.3390/curroncol28060426
Durable Response to Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, and Prednisone (BV-CHP) in a Patient with CD30-Positive PTCL Arising as a Post-Transplant Lymphoproliferative Disorder (PTLD)
Abstract
T-cell PTLDs are lymphoid proliferations that develop in recipients of SOT or allogeneic HSCT. They carry an extremely poor prognosis with a reported median survival of only 6 months. The infrequency with which they are encountered makes treatment a challenge due to the lack of prospective trials to guide management. The significantly higher risk of morbidity and mortality in T-cell PTLD, compared to B-cell PTLD, underscores the challenge of treating these patients and the need for new therapeutic options. Brentuximab vedotin, an ADC targeting CD30, is FDA-approved in combination with CHP as front-line treatment for patients with CD30 expressing PTCL. Herein we report a case of CD30-positive T-cell PTLD that was successfully treated with BV-CHP, suggesting the added value of the addition of BV to chemotherapy, contributing to our patient's long and ongoing progression-free survival. To our knowledge, this is the first documented case of successful treatment using BV-CHP for a CD30-positive, EBV-negative, late T-cell PTLD.
Keywords: BV-CHP; CD30; NOS; T-cell; brentuximab; peripheral T-cell lymphoma; post-transplant lymphoproliferative disorders.
Conflict of interest statement
The authors declare no conflict of interest.
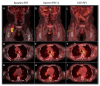
Figures

References
-
- Caillard S., Porcher R., Provot F., Dantal J., Choquet S., Durrbach A., Morelon E., Moal V., Janbon B., Alamartine E., et al. Post-transplantation lymphoproliferative disorder after kidney transplantation: Report of a nationwide French registry and the development of a new prognostic score. Am. J. Clin. Oncol. 2013;31:1302–1309. doi: 10.1200/JCO.2012.43.2344. - DOI - PubMed
-
- Montanari F., Bhagat G., Clark-Garvey S., Seshan V., Zain J., Diefenbach C., Mccormick E., Crook M., Conroy M., O’connor O.A. Monomorphic T-cell post-transplant lymphoproliferative disorders exhibit markedly inferior outcomes compared to monomorphic B-cell post-transplant lymphoproliferative disorders. Leuk. Lymphoma. 2010;51:1761–1764. doi: 10.3109/10428194.2010.500436. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

