Stink Bug Communication and Signal Detection in a Plant Environment
- PMID: 34940147
- PMCID: PMC8705670
- DOI: 10.3390/insects12121058
Stink Bug Communication and Signal Detection in a Plant Environment
Abstract
Plants influenced the evolution of plant-dwelling stink bugs' systems underlying communication with chemical and substrate-borne vibratory signals. Plant volatiles provides cues that increase attractiveness or interfere with the probability of finding a mate in the field. Mechanical properties of herbaceous hosts and associated plants alter the frequency, amplitude, and temporal characteristics of stink bug species and sex-specific vibratory signals. The specificity of pheromone odor tuning has evolved through highly specific odorant receptors located within the receptor membrane. The narrow-band low-frequency characteristics of the signals produced by abdomen vibration and the frequency tuning of the highly sensitive subgenual organ vibration receptors match with filtering properties of the plants enabling optimized communication. A range of less sensitive mechanoreceptors, tuned to lower vibration frequencies, detect signals produced by other mechanisms used at less species-specific levels of communication in a plant environment. Whereas the encoding of frequency-intensity and temporal parameters of stink bug vibratory signals is relatively well investigated at low levels of processing in the ventral nerve cord, processing of this information and its integration with other modalities at higher neuronal levels still needs research attention.
Keywords: Pentatominae stink bugs; biotremology; communication; evolution; host plants; plant-dwelling insects; sensory system; signals; transmission medium.
Conflict of interest statement
The authors declare no conflict of interests.
Figures


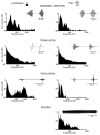
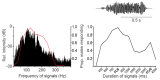


Similar articles
-
Stink bug interaction with host plants during communication.J Insect Physiol. 2008 Jul;54(7):1113-24. doi: 10.1016/j.jinsphys.2008.06.004. Epub 2008 Jun 26. J Insect Physiol. 2008. PMID: 18634798 Review.
-
Tremulatory and abdomen vibration signals enable communication through air in the stink bug Euschistus heros.PLoS One. 2013;8(2):e56503. doi: 10.1371/journal.pone.0056503. Epub 2013 Feb 27. PLoS One. 2013. PMID: 23460803 Free PMC article.
-
Communication with substrate-borne signals in small plant-dwelling insects.Annu Rev Entomol. 2003;48:29-50. doi: 10.1146/annurev.ento.48.091801.112605. Epub 2002 Jun 4. Annu Rev Entomol. 2003. PMID: 12414736 Review.
-
Inhibitory Copulation Effect of Vibrational Rival Female Signals of Three Stink Bug Species as a Tool for Mating Disruption.Insects. 2021 Feb 18;12(2):177. doi: 10.3390/insects12020177. Insects. 2021. PMID: 33670780 Free PMC article.
-
Tuning of host plants with vibratory songs of Nezara viridula L (Heteroptera: Pentatomidae).J Exp Biol. 2005 Apr;208(Pt 8):1481-8. doi: 10.1242/jeb.01557. J Exp Biol. 2005. PMID: 15802672
Cited by
-
The post-diapause vibrational behavior, motility, and survival of the brown marmorated stink bug Halyomorpha halys (Stål) adults at different temperatures.Sci Rep. 2024 Jan 12;14(1):1198. doi: 10.1038/s41598-023-50480-y. Sci Rep. 2024. PMID: 38216589 Free PMC article.
References
-
- Grazia J., Schwertner L.A. Stink Bug Classification, Phylogeny, Biology and Reproductive Behavior. In: Čokl A., Borges M., editors. Biorational Control Based on Communication Processes. 1st ed. Volume 1. CRC Press; Boca Raton, FL, USA: 2017. pp. 1–30.
-
- Grazia J., Panizzi A.R., Schwertner L.A., Campos L.A., Garbelotto T.A. Short Views on Insect Genomics and Proteomics. Springer; Singapore: 2015. True Bugs (Heteroptera) of the Neotropics; pp. 681–756.
-
- Panizzi A.R., Lucini T. Biorational Control Based on Communication Processes. Volume 5. CRC Press; Boca Raton, FL, USA: 2017. Host plant-stink bug relationships; pp. 31–85.
-
- Laumann R.A., Bottura Maccagnan D.H., Čokl A. Biorational Control Based on Communication Processes. Volume 1. CRC Press; Boca Raton, FL, USA: 2017. Use of Vibratory Signals for Stink Bug Monitoring and Control; pp. 226–245.
-
- Panizzi A.R., McPherson J.E., James D.G., Javahery M., McPherson R.M. Stink bugs (Pentatomidae) In: Schaefer C.W., Panizzi A.R., editors. Heteroptera of Economic Importance. 1st ed. CRC Press Taylor & Francis Group; Boca Raton, FL, USA: 2000. pp. 421–474.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

