Genomic Variants and Multilevel Regulation of ABCA1, ABCG1, and SCARB1 Expression in Atherogenesis
- PMID: 34940525
- PMCID: PMC8707585
- DOI: 10.3390/jcdd8120170
Genomic Variants and Multilevel Regulation of ABCA1, ABCG1, and SCARB1 Expression in Atherogenesis
Abstract
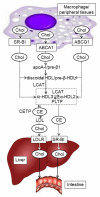
Atheroprotective properties of human plasma high-density lipoproteins (HDLs) are determined by their involvement in reverse cholesterol transport (RCT) from the macrophage to the liver. ABCA1, ABCG1, and SR-BI cholesterol transporters are involved in cholesterol efflux from macrophages to lipid-free ApoA-I and HDL as a first RCT step. Molecular determinants of RCT efficiency that may possess diagnostic and therapeutic meaning remain largely unknown. This review summarizes the progress in studying the genomic variants of ABCA1, ABCG1, and SCARB1, and the regulation of their function at transcriptional and post-transcriptional levels in atherosclerosis. Defects in the structure and function of ABCA1, ABCG1, and SR-BI are caused by changes in the gene sequence, such as single nucleotide polymorphism or various mutations. In the transcription initiation of transporter genes, in addition to transcription factors, long noncoding RNA (lncRNA), transcription activators, and repressors are also involved. Furthermore, transcription is substantially influenced by the methylation of gene promoter regions. Post-transcriptional regulation involves microRNAs and lncRNAs, including circular RNAs. The potential biomarkers and targets for atheroprotection, based on molecular mechanisms of expression regulation for three transporter genes, are also discussed in this review.
Keywords: ABCA1; ABCG1; SCARB1; atherosclerosis; cholesterol efflux; gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

