New Insights to the Crosstalk between Vascular and Bone Tissue in Chronic Kidney Disease-Mineral and Bone Disorder
- PMID: 34940607
- PMCID: PMC8708186
- DOI: 10.3390/metabo11120849
New Insights to the Crosstalk between Vascular and Bone Tissue in Chronic Kidney Disease-Mineral and Bone Disorder
Abstract
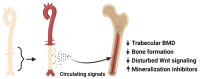
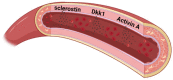
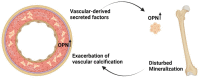
Vasculature plays a key role in bone development and the maintenance of bone tissue throughout life. The two organ systems are not only linked in normal physiology, but also in pathophysiological conditions. The chronic kidney disease-mineral and bone disorder (CKD-MBD) is still the most serious complication to CKD, resulting in increased morbidity and mortality. Current treatment therapies aimed at the phosphate retention and parathyroid hormone disturbances fail to reduce the high cardiovascular mortality in CKD patients, underlining the importance of other factors in the complex syndrome. This review will focus on vascular disease and its interplay with bone disorders in CKD. It will present the very late data showing a direct effect of vascular calcification on bone metabolism, indicating a vascular-bone tissue crosstalk in CKD. The calcified vasculature not only suffers from the systemic effects of CKD but seems to be an active player in the CKD-MBD syndrome impairing bone metabolism and might be a novel target for treatment and prevention.
Keywords: TGF-β signaling; Wnt pathway; activin A; dickkopf-1; renal osteodystrophy; sclerostin; tissue crosstalk; uremic vasculopathy; vascular calcification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Medical

