Cardiac Metabolism and Contractile Function in Mice with Reduced Trans-Endothelial Fatty Acid Transport
- PMID: 34940647
- PMCID: PMC8706312
- DOI: 10.3390/metabo11120889
Cardiac Metabolism and Contractile Function in Mice with Reduced Trans-Endothelial Fatty Acid Transport
Abstract
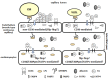
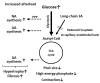
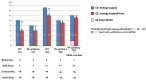
The heart is a metabolic omnivore that combusts a considerable amount of energy substrates, mainly long-chain fatty acids (FAs) and others such as glucose, lactate, ketone bodies, and amino acids. There is emerging evidence that muscle-type continuous capillaries comprise the rate-limiting barrier that regulates FA uptake into cardiomyocytes. The transport of FAs across the capillary endothelium is composed of three major steps-the lipolysis of triglyceride on the luminal side of the endothelium, FA uptake by the plasma membrane, and intracellular FA transport by cytosolic proteins. In the heart, impaired trans-endothelial FA (TEFA) transport causes reduced FA uptake, with a compensatory increase in glucose use. In most cases, mice with reduced FA uptake exhibit preserved cardiac function under unstressed conditions. When the workload is increased, however, the total energy supply relative to its demand (estimated with pool size in the tricarboxylic acid (TCA) cycle) is significantly diminished, resulting in contractile dysfunction. The supplementation of alternative fuels, such as medium-chain FAs and ketone bodies, at least partially restores contractile dysfunction, indicating that energy insufficiency due to reduced FA supply is the predominant cause of cardiac dysfunction. Based on recent in vivo findings, this review provides the following information related to TEFA transport: (1) the mechanisms of FA uptake by the heart, including TEFA transport; (2) the molecular mechanisms underlying the induction of genes associated with TEFA transport; (3) in vivo cardiac metabolism and contractile function in mice with reduced TEFA transport under unstressed conditions; and (4) in vivo contractile dysfunction in mice with reduced TEFA transport under diseased conditions, including an increased afterload and streptozotocin-induced diabetes.
Keywords: TCA cycle; capillary endothelium; cardiac metabolism; contractile function; fatty acid; glucose; pool size; trans-endothelial fatty acid transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Taegtmeyer H., Young M.E., Lopaschuk G.D., Abel E.D., Brunengraber H., Darley-Usmar V., Des Rosiers C., Gerszten R., Glatz J.F., Griffin J.L., et al. Assessing Cardiac Metabolism: A Scientific Statement from the American Heart Association. Circ. Res. 2016;118:1659–1701. doi: 10.1161/RES.0000000000000097. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

