ACE Inhibitory Peptide from Skin Collagen Hydrolysate of Takifugu bimaculatus as Potential for Protecting HUVECs Injury
- PMID: 34940654
- PMCID: PMC8703921
- DOI: 10.3390/md19120655
ACE Inhibitory Peptide from Skin Collagen Hydrolysate of Takifugu bimaculatus as Potential for Protecting HUVECs Injury
Abstract
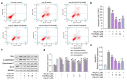
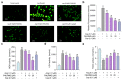
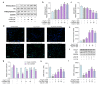
Angiotensin-I-converting enzyme (ACE) is a crucial enzyme or receptor that catalyzes the generation of potent vasopressor angiotensin II (Ang II). ACE inhibitory peptides from fish showed effective ACE inhibitory activity. In this study, we reported an ACE inhibitory peptide from Takifugu bimaculatus (T. bimaculatus), which was obtained by molecular docking with acid-soluble collagen (ASC) hydrolysate of T. bimaculatus. The antihypertensive effects and potential mechanism were conducted using Ang-II-induced human umbilical vein endothelial cells (HUVECs) as a model. The results showed that FNLRMQ alleviated the viability and facilitated apoptosis of Ang-II-induced HUVECs. Further research suggested that FNLRMQ may protect Ang-II-induced endothelial injury by regulating Nrf2/HO-1 and PI3K/Akt/eNOS signaling pathways. This study, herein, reveals that collagen peptide FNLRMQ could be used as a potential candidate compound for antihypertensive treatment, and could provide scientific evidence for the high-value utilization of marine resources including T. bimaculatus.
Keywords: HUVECs; Takifugu bimaculatus; antihypertension; skin collagen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

