Final Overall Survival and Molecular Analysis in IMmotion151, a Phase 3 Trial Comparing Atezolizumab Plus Bevacizumab vs Sunitinib in Patients With Previously Untreated Metastatic Renal Cell Carcinoma
- PMID: 34940781
- PMCID: PMC8855230
- DOI: 10.1001/jamaoncol.2021.5981
Final Overall Survival and Molecular Analysis in IMmotion151, a Phase 3 Trial Comparing Atezolizumab Plus Bevacizumab vs Sunitinib in Patients With Previously Untreated Metastatic Renal Cell Carcinoma
Abstract
Importance: Interim analyses of the IMmotion151 trial (A Study of Atezolizumab in Combination With Bevacizumab Versus Sunitinib in Participants With Untreated Advanced Renal Cell Carcinoma) reported improved progression-free survival (PFS) for patients with programmed death ligand 1-positive (PD-L1+) metastatic renal cell carcinoma (mRCC) receiving the PD-L1 inhibitor atezolizumab plus the vascular endothelial growth factor (VEGF) inhibitor bevacizumab vs the receptor tyrosine kinase inhibitor sunitinib. Overall survival (OS) results were immature at interim analyses.
Objective: To report the final OS results, safety, and exploratory biomarker analyses of the association of transcriptomic subgroups with OS in the IMmotion151 trial.
Design, setting, and participants: IMmotion151 was a multicenter, open-label, phase 3 randomized clinical trial that compared the efficacy and safety of atezolizumab plus bevacizumab vs sunitinib in patients with untreated mRCC. IMmotion151 included patients from 152 academic medical centers and community oncology practices in 21 countries. Adult patients with mRCC with components of clear cell or sarcomatoid histologic features, measurable disease (according to Response Evaluation Criteria in Solid Tumors, version 1.1), adequate performance status, hematologic and end organ function, and tumor tissue available for PD-L1 testing were included. IMmotion151 was initiated on May 20, 2015, and the study is ongoing. This final analysis was performed from May 20, 2015, to February 14, 2020.
Interventions: Receipt of 1200 mg of intravenous (IV) atezolizumab every 3 weeks and 15 mg/kg of IV bevacizumab every 3 weeks or 50 mg orally once daily of sunitinib (4 weeks on and 2 weeks off).
Main outcomes and measures: The coprimary end points were PFS (previously reported) in patients with PD-L1+ disease and OS in the intention-to-treat population. Additional exploratory outcomes included OS in the PD-L1+ population, association with transcriptomic subgroups, and safety.
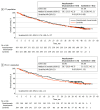
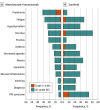
Results: The IMmotion151 trial assessed 915 patients with metastatic renal cell carcinoma. Mean (IQR) age was 62 (56-69) years for patients receiving atezolizumab plus bevacizumab and 60 (54-66) years for patients receiving sunitinib; 669 (73.1%) were male and 246 (26.9%) were female. The final analysis showed similar median OS in patients receiving atezolizumab plus bevacizumab vs sunitinib in the intention-to-treat (36.1 vs 35.3 months) and PD-L1+ (38.7 vs 31.6 months) populations. No new safety signals were reported. The additional exploratory outcome of atezolizumab plus bevacizumab vs sunitinib showed improved median OS trends in patients whose tumors were characterized by T-effector/proliferative, proliferative, or small nucleolar RNA transcriptomic profiles (35.4 vs 21.2 months; hazard ratio, 0.70; 95% CI, 0.50-0.98).
Conclusions and relevance: The primary end point of PFS was met at interim analyses, although no improvement in OS was observed with atezolizumab plus bevacizumab at the final analysis. Biomarker analyses provided insight into which patients with mRCC may benefit from combined anti-PD-L1 and anti-VEGF therapy.
Trial registration: ClinicalTrials.gov Identifier: NCT02420821.
Conflict of interest statement
Figures

References
-
- Rini BI, Powles T, Atkins MB, et al. ; IMmotion151 Study Group . Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404-2415. doi:10.1016/S0140-6736(19)30723-8 - DOI - PubMed
-
- Motzer RJ, Powles T, Atkins MB, et al. . IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2018;36(6)(suppl):578-578. doi:10.1200/JCO.2018.36.6_suppl.578 - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

