Fixational eye movements following concussion
- PMID: 34940825
- PMCID: PMC8709928
- DOI: 10.1167/jov.21.13.11
Fixational eye movements following concussion
Abstract
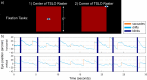
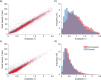
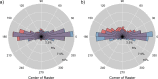
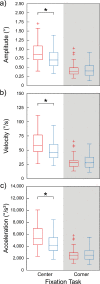
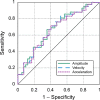
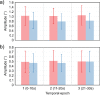
The purpose of this study was to evaluate fixational eye movements (FEMs) with high spatial and temporal resolution following concussion, where oculomotor symptoms and impairments are common. Concussion diagnosis was determined using current consensus guidelines. A retinal eye-tracking device, the tracking scanning laser ophthalmoscope (TSLO), was used to measure FEMs in adolescents and young adults following a concussion and in an unaffected control population. FEMs were quantified in two fixational paradigms: (1) when fixating on the center, or (2) when fixating on the corner of the TSLO imaging raster. Fixational saccade amplitude in recent concussion patients (≤ 21 days) was significantly greater, on average, in the concussion group (mean = 1.03°; SD = 0.36°) compared with the controls (mean = 0.82°; SD = 0.31°), when fixating on the center of the imaging raster (t = 2.87, df = 82, p = 0.005). These fixational saccades followed the main sequence and therefore also had greater peak velocity (t = 2.86, df = 82, p = 0.006) and peak acceleration (t = 2.80, df = 82, p = 0.006). These metrics significantly differentiated concussed from controls (AUC = 0.67-0.68, minimum p = 0.005). No group differences were seen for the drift metrics in either task or for any of the FEMs metrics in the corner-of-raster fixation task. Fixational saccade amplitudes were significantly different in the concussion group, but only when fixating on the center of the raster. This task specificity suggests that task optimization may improve differentiation and warrants further study. FEMs measured in the acute-to-subacute period of concussion recovery may provide a quick (<3 minutes), objective, sensitive, and accurate ocular dysfunction assessment. Future work should assess the impact of age, mechanism of injury, and post-concussion recovery on FEM alterations following concussion.
Figures
References
-
- Collins, M. W., Kontos, A. P., Okonkwo, D. O., Almquist, J., Bailes, J., Barisa, M., …, Zafonte, R. (2016). Statements of agreement from the Targeted Evaluation and Active Management (TEAM) Approaches to Treating Concussion Meeting held in Pittsburgh, October 15–16, 2015. Neurosurgery, 79(6), 912–929, 10.1227/NEU.0000000000001447. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

