Oxygen flux from capillary to mitochondria: integration of contemporary discoveries
- PMID: 34940908
- PMCID: PMC8890444
- DOI: 10.1007/s00421-021-04854-7
Oxygen flux from capillary to mitochondria: integration of contemporary discoveries
Abstract
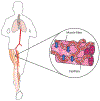
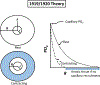
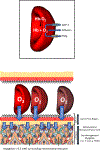
Resting humans transport ~ 100 quintillion (1018) oxygen (O2) molecules every second to tissues for consumption. The final, short distance (< 50 µm) from capillary to the most distant mitochondria, in skeletal muscle where exercising O2 demands may increase 100-fold, challenges our understanding of O2 transport. To power cellular energetics O2 reaches its muscle mitochondrial target by dissociating from hemoglobin, crossing the red cell membrane, plasma, endothelial surface layer, endothelial cell, interstitial space, myocyte sarcolemma and a variable expanse of cytoplasm before traversing the mitochondrial outer/inner membranes and reacting with reduced cytochrome c and protons. This past century our understanding of O2's passage across the body's final O2 frontier has been completely revised. This review considers the latest structural and functional data, challenging the following entrenched notions: (1) That O2 moves freely across blood cell membranes. (2) The Krogh-Erlang model whereby O2 pressure decreases systematically from capillary to mitochondria. (3) Whether intramyocyte diffusion distances matter. (4) That mitochondria are separate organelles rather than coordinated and highly plastic syncytia. (5) The roles of free versus myoglobin-facilitated O2 diffusion. (6) That myocytes develop anoxic loci. These questions, and the intriguing notions that (1) cellular membranes, including interconnected mitochondrial membranes, act as low resistance conduits for O2, lipids and H+-electrochemical transport and (2) that myoglobin oxy/deoxygenation state controls mitochondrial oxidative function via nitric oxide, challenge established tenets of muscle metabolic control. These elements redefine muscle O2 transport models essential for the development of effective therapeutic countermeasures to pathological decrements in O2 supply and physical performance.
Keywords: Aquaporin channels; Capillary function; Exercise limitation; Mitochondrial structure; Muscle anoxia; Myoglobin; Perfusive and diffusive oxygen conductances; Rhesus channels.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Figures
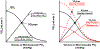



Comment in
-
Comment on Poole et al (2022) review on oxygen flux from capillaries to mitochondria.Eur J Appl Physiol. 2022 Jan;122(1):5-6. doi: 10.1007/s00421-021-04872-5. Epub 2021 Dec 18. Eur J Appl Physiol. 2022. PMID: 34921605 No abstract available.
Similar articles
-
Capillary-Mitochondrial Oxygen Transport in Muscle: Paradigm Shifts.Function (Oxf). 2023 Mar 16;4(3):zqad013. doi: 10.1093/function/zqad013. eCollection 2023. Function (Oxf). 2023. PMID: 37168497 Free PMC article. Review.
-
August Krogh's theory of muscle microvascular control and oxygen delivery: a paradigm shift based on new data.J Physiol. 2020 Oct;598(20):4473-4507. doi: 10.1113/JP279223. Epub 2020 Sep 12. J Physiol. 2020. PMID: 32918749 Review.
-
Dynamics of muscle microcirculatory and blood-myocyte O(2) flux during contractions.Acta Physiol (Oxf). 2011 Jul;202(3):293-310. doi: 10.1111/j.1748-1716.2010.02246.x. Epub 2011 Mar 1. Acta Physiol (Oxf). 2011. PMID: 21199399 Free PMC article. Review.
-
Myoglobin content and oxygen diffusion: model analysis of horse and steer muscle.Am J Physiol. 1996 Dec;271(6 Pt 1):C2027-36. doi: 10.1152/ajpcell.1996.271.6.C2027. Am J Physiol. 1996. PMID: 8997205
-
August Krogh: Muscle capillary function and oxygen delivery.Comp Biochem Physiol A Mol Integr Physiol. 2021 Mar;253:110852. doi: 10.1016/j.cbpa.2020.110852. Epub 2020 Nov 24. Comp Biochem Physiol A Mol Integr Physiol. 2021. PMID: 33242636 Free PMC article. Review.
Cited by
-
The Oxygen Cascade from Atmosphere to Mitochondria as a Tool to Understand the (Mal)adaptation to Hypoxia.Int J Mol Sci. 2023 Feb 12;24(4):3670. doi: 10.3390/ijms24043670. Int J Mol Sci. 2023. PMID: 36835089 Free PMC article. Review.
-
Cardiac output limits maximal oxygen consumption, but what limits maximal cardiac output?Exp Physiol. 2025 May;110(5):666-674. doi: 10.1113/EP091594. Epub 2025 Apr 7. Exp Physiol. 2025. PMID: 40193294 Free PMC article. Review.
-
Impact of capillary and sarcolemmal proximity on mitochondrial structure and energetic function in skeletal muscle.J Physiol. 2024 May;602(9):1967-1986. doi: 10.1113/JP286246. Epub 2024 Apr 2. J Physiol. 2024. PMID: 38564214 Free PMC article.
-
The role of the microcirculation and integrative cardiovascular physiology in the pathogenesis of ICU-acquired weakness.Front Physiol. 2023 May 10;14:1170429. doi: 10.3389/fphys.2023.1170429. eCollection 2023. Front Physiol. 2023. PMID: 37234410 Free PMC article. Review.
-
Cancer Therapy and Exercise Intolerance: The Heart Is But a Part: JACC: CardioOncology State-of-the-Art Review.JACC CardioOncol. 2024 Jun 4;6(4):496-513. doi: 10.1016/j.jaccao.2024.04.006. eCollection 2024 Aug. JACC CardioOncol. 2024. PMID: 39239327 Free PMC article. Review.
References
-
- Aguirre E, Rodríguez-Juárez F, Bellelli A, Gnaiger E, Cadenas S (2010) Kinetic model of the inhibition of respiration by endogenous nitric oxide in intact cells. Biochim Biophys Acta 1797:557–565. - PubMed
-
- Al-Shammari AA, Kissane RWP, Holbek S, Mackey AL, Andersen TR, Gaffney EA, Kjaer M, Egginton S (2019) Integrated method for quantitative morphometry and oxygen transport modeling in striated muscle. J Appl Physiol (1985). 126:544–557. - PubMed
-
- Angleys H, Østergaard L (2020) Krogh’s capillary recruitment hypothesis, 100 years on: Is the opening of previously closed capillaries necessary to ensure muscle oxygenation during exercise? Am J Physiol Heart Circ Physiol 318:H425–H447. - PubMed
-
- Arihara K, Cassens RG, Greaser ML, Luchansky JB, Mozdziak PE (1995) Localization of metmyoglobin-reducing enzyme (NADH-cytochrome b(5) reductase) system components in bovine skeletal muscle. Meat Sci 39:205–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

