Methods to Generate Innovative Research Ideas and Improve Patient and Public Involvement in Modern Epidemiological Research: Review, Patient Viewpoint, and Guidelines for Implementation of a Digital Cohort Study
- PMID: 34941554
- PMCID: PMC8738987
- DOI: 10.2196/25743
Methods to Generate Innovative Research Ideas and Improve Patient and Public Involvement in Modern Epidemiological Research: Review, Patient Viewpoint, and Guidelines for Implementation of a Digital Cohort Study
Abstract
Background: Patient and public involvement (PPI) in research aims to increase the quality and relevance of research by incorporating the perspective of those ultimately affected by the research. Despite these potential benefits, PPI is rarely included in epidemiology protocols.
Objective: The aim of this study is to provide an overview of methods used for PPI and offer practical recommendations for its efficient implementation in epidemiological research.
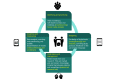
Methods: We conducted a review on PPI methods. We mirrored it with a patient advocate's viewpoint about PPI. We then identified key steps to optimize PPI in epidemiological research based on our review and the viewpoint of the patient advocate, taking into account the identification of barriers to, and facilitators of, PPI. From these, we provided practical recommendations to launch a patient-centered cohort study. We used the implementation of a new digital cohort study as an exemplary use case.
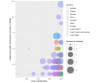
Results: We analyzed data from 97 studies, of which 58 (60%) were performed in the United Kingdom. The most common methods were workshops (47/97, 48%); surveys (33/97, 34%); meetings, events, or conferences (28/97, 29%); focus groups (25/97, 26%); interviews (23/97, 24%); consensus techniques (8/97, 8%); James Lind Alliance consensus technique (7/97, 7%); social media analysis (6/97, 6%); and experience-based co-design (3/97, 3%). The viewpoint of a patient advocate showed a strong interest in participating in research. The most usual PPI modalities were research ideas (60/97, 62%), co-design (42/97, 43%), defining priorities (31/97, 32%), and participation in data analysis (25/97, 26%). We identified 9 general recommendations and 32 key PPI-related steps that can serve as guidelines to increase the relevance of epidemiological studies.
Conclusions: PPI is a project within a project that contributes to improving knowledge and increasing the relevance of research. PPI methods are mainly used for idea generation. On the basis of our review and case study, we recommend that PPI be included at an early stage and throughout the research cycle and that methods be combined for generation of new ideas. For e-cohorts, the use of digital tools is essential to scale up PPI. We encourage investigators to rely on our practical recommendations to extend PPI in future epidemiological studies.
Keywords: co-design; digital cohort study; digital epidemiology; focus groups; mobile phone; patient and public involvement; social media; surveys; workshops.
©Gloria A Aguayo, Catherine Goetzinger, Renza Scibilia, Aurélie Fischer, Till Seuring, Viet-Thi Tran, Philippe Ravaud, Tamás Bereczky, Laetitia Huiart, Guy Fagherazzi. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 23.12.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Tips sheet: recruiting members of the public to get involved in research funding and commissioning processes. INVOLVE - National Institute for Health Research. 2012. [2019-11-20]. http://www.invo.org.uk/wp-content/uploads/2012/04/Recruitment-tips-sheet... .
-
- What is patient and public involvement and public engagement? National Institute for Health Research. 2020. [2021-12-07]. https://www.spcr.nihr.ac.uk/PPI/what-is-patient-and-public-involvement-a... .
-
- Trish Greenhalgh: towards an institute for patient-led research. The BMJ Opinion. 2019. [2021-12-07]. https://blogs.bmj.com/bmj/2019/11/12/trisha-greenhalgh-towards-an-instit...
-
- Crocker JC, Ricci-Cabello I, Parker A, Hirst JA, Chant A, Petit-Zeman S, Evans D, Rees S. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. Br Med J. 2018 Nov 28;363:k4738. doi: 10.1136/bmj.k4738. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=30487232 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

