Diagnostics for COVID-19: moving from pandemic response to control
- PMID: 34942102
- PMCID: PMC8687671
- DOI: 10.1016/S0140-6736(21)02346-1
Diagnostics for COVID-19: moving from pandemic response to control
Abstract
Diagnostics have proven to be crucial to the COVID-19 pandemic response. There are three major methods for the detection of SARS-CoV-2 infection and their role has evolved during the course of the pandemic. Molecular tests such as PCR are highly sensitive and specific at detecting viral RNA, and are recommended by WHO for confirming diagnosis in individuals who are symptomatic and for activating public health measures. Antigen rapid detection tests detect viral proteins and, although they are less sensitive than molecular tests, have the advantages of being easier to do, giving a faster time to result, of being lower cost, and able to detect infection in those who are most likely to be at risk of transmitting the virus to others. Antigen rapid detection tests can be used as a public health tool for screening individuals at enhanced risk of infection, to protect people who are clinically vulnerable, to ensure safe travel and the resumption of schooling and social activities, and to enable economic recovery. With vaccine roll-out, antibody tests (which detect the host's response to infection or vaccination) can be useful surveillance tools to inform public policy, but should not be used to provide proof of immunity, as the correlates of protection remain unclear. All three types of COVID-19 test continue to have a crucial role in the transition from pandemic response to pandemic control.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
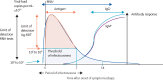
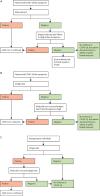
References
-
- WHO WHO Director-General's opening remarks at the media briefing on COVID-19—16 March 2020. https://www.who.int/director-general/speeches/detail/who-director-genera...
-
- FIND Rapid diagnostic tests for COVID-19. May 18, 2020. https://www.finddx.org/wp-content/uploads/2020/05/FIND_COVID-19_RDTs_18....
-
- Nkengasong J. Let Africa into the market for COVID-19 diagnostics. Nature. 2020;580:565. - PubMed
-
- WHO Emergency use listing of in-vitro diagnostics. 2020. https://www.who.int/teams/regulation-prequalification/eul/in-vitro-emerg...
-
- US Food and Drug Administration In vitro diagnostics EUAs. Nov 29, 2021. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

