A Single Dose of Ginkgo biloba Extract Induces Gene Expression of Hypothalamic Anorexigenic Effectors in Male Rats
- PMID: 34942904
- PMCID: PMC8699374
- DOI: 10.3390/brainsci11121602
A Single Dose of Ginkgo biloba Extract Induces Gene Expression of Hypothalamic Anorexigenic Effectors in Male Rats
Abstract
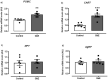
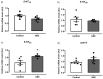
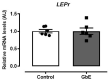
Previous studies have shown that Ginkgo biloba extract (GbE) reduces food intake and body mass gain and regulates proteins related to lipid metabolism in obese rats. In ovariectomized rats, GbE restored the hippocampal and hypothalamic serotonergic system activity, favoring the spontaneous feeding decrement. Considering the promising hypophagic effect of GbE, this study aimed to investigate the effect of a single acute dose on hypothalamic pathways that regulate feeding behavior in male rats. Four-month-old Wistar male rats received either a single acute oral GbE dose (500 mg/kg) or vehicle. Food intake and body mass were measured after 1, 4, 12, and 24 h. Rats were euthanized, and hypothalami were removed for mRNA quantification of anorexigenic (POMC/CART) and orexigenic (AgRP/NPY) neuropeptides, leptin/serotonin receptors (5HT1A, 5HT1B, 5HT2C), and serotonin transporters. We also investigated POMC, 5-HT1B, and 5-HT2C protein levels. A single acute GbE dose induced the hypothalamic POMC, CART, and 5-HT2C gene expression but failed to modify orexigenic effectors. No alterations in food intake, body mass, and hypothalamic protein levels were observed. In summary, the present findings demonstrate the rapid stimulation of pivotal hypothalamic anorexigenic pathways in response to a single GbE administration, reinforcing the GbE hypophagic activity. However, more studies are necessary to evaluate its potential as an appetite modulator.
Keywords: food intake GbE; hypothalamic neuropeptides; serotonergic system.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding this publication.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

