Regioselective and Stereoselective Epoxidation of n-3 and n-6 Fatty Acids by Fungal Peroxygenases
- PMID: 34942990
- PMCID: PMC8698580
- DOI: 10.3390/antiox10121888
Regioselective and Stereoselective Epoxidation of n-3 and n-6 Fatty Acids by Fungal Peroxygenases
Abstract
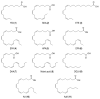
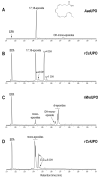
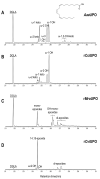
Epoxide metabolites from n-3 and n-6 polyunsaturated fatty acids arouse interest thanks to their physiological and pharmacological activities. Their chemical synthesis has significant drawbacks, and enzymes emerge as an alternative with potentially higher selectivity and greener nature. Conversion of eleven eicosanoid, docosanoid, and other n-3/n-6 fatty acids into mono-epoxides by fungal unspecific peroxygenases (UPOs) is investigated, with emphasis on the Agrocybe aegerita (AaeUPO) and Collariella virescens (rCviUPO) enzymes. GC-MS revealed the strict regioselectivity of the n-3 and n-6 reactions with AaeUPO and rCviUPO, respectively, yielding 91%-quantitative conversion into mono-epoxides at the last double bond. Then, six of these mono-epoxides were obtained at mg-scale, purified and further structurally characterized by 1H, 13C and HMBC NMR. Moreover, chiral HPLC showed that the n-3 epoxides were also formed (by AaeUPO) with total S/R enantioselectivity (ee > 99%) while the n-6 epoxides (from rCviUPO reactions) were formed in nearly racemic mixtures. The high regio- and enantioselectivity of several of these reactions unveils the synthetic utility of fungal peroxygenases in fatty acid epoxidation.
Keywords: NMR; bioactive compounds; chiral HPLC; epoxylipids; omega 3 (n-3) fatty acids; omega 6 (n-6) fatty acids; polyunsaturated fatty acids; regioselective synthesis; stereoselective synthesis; unspecific peroxygenases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



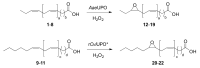
References
-
- Darwesh A.M., Bassiouni W., Adebesin A.M., Mohammad A.S., Falck J.R., Seubert J.M. A synthetic epoxydocosapentaenoic acid analogue ameliorates cardiac ischemia/reperfusion injury: The involvement of the sirtuin 3-NLRP3 pathway. Int. J. Mol. Sci. 2020;21:5261. doi: 10.3390/ijms21155261. - DOI - PMC - PubMed
-
- Chen X., Li Z., Zhang B., Hu R., Li J., Feng M., Yao W., Zhang C., Wan L., Zhang Y. Alleviation of mechanical allodynia by 14,15-epoxyeicosatrienoic acid in a central poststroke pain model: Possible role of allopregnanolone and d-subunit-containing gamma-aminobutyric acid A receptors. J. Pain. 2019;20:577–591. doi: 10.1016/j.jpain.2018.11.006. - DOI - PubMed
-
- Leineweber C.G., Pietzner A., Zhang I.W., Blessin U.B., Rothe M., Schott E., Schebb N.H., Weylandt K.H. Assessment of the effect of sorafenib on omega-6 and omega-3 epoxyeicosanoid formation in patients with hepatocellular carcinoma. Int. J. Mol. Sci. 2020;21:1875. doi: 10.3390/ijms21051875. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

