Ethyl Acetate Fractions of Papaver rhoeas L. and Papaver nudicaule L. Exert Antioxidant and Anti-Inflammatory Activities
- PMID: 34942995
- PMCID: PMC8750608
- DOI: 10.3390/antiox10121895
Ethyl Acetate Fractions of Papaver rhoeas L. and Papaver nudicaule L. Exert Antioxidant and Anti-Inflammatory Activities
Abstract
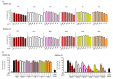
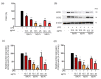
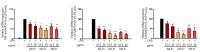
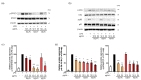
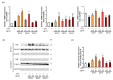
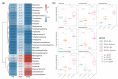
Abnormal inflammation and oxidative stress are involved in various diseases. Papaver rhoeas L. possesses various pharmacological activities, and a previously reported analysis of the anti-inflammatory effect of P. nudicaule ethanol extracts and alkaloid profiles of the plants suggest isoquinoline alkaloids as potential pharmacologically active compounds. Here, we investigated anti-inflammatory and antioxidant activities of ethyl acetate (EtOAc) fractions of P. nudicaule and P. rhoeas extracts in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. EtOAc fractions of P. nudicaule and P. rhoeas compared to their ethanol extracts showed less toxicity but more inhibitory activity against LPS-induced nitric oxide production. Moreover, EtOAc fractions lowered the LPS-induced production of proinflammatory molecules and cytokines and inhibited LPS-activated STAT3 and NF-κB, and additionally showed significant free radical scavenging activity and decreased LPS-induced reactive oxygen species and oxidized glutathione. EtOAc fractions of P. nudicaule increased the expression of HO-1, GCLC, NQO-1, and Nrf2 in LPS-stimulated cells and that of P. rhoeas enhanced NQO-1. Furthermore, metabolomic and biochemometric analyses of ethanol extracts and EtOAc fractions indicated that EtOAc fractions of P. nudicaule and P. rhoeas have potent anti-inflammatory and antioxidant activities, further suggesting that alkaloids in EtOAc fractions are potent active molecules of tested plants.
Keywords: NF-κB; Nrf2; Papaver nudicaule; Papaver rhoeas; STAT3; anti-inflammatory; antioxidant.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the study design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

