Mito-TIPTP Increases Mitochondrial Function by Repressing the Rubicon-p22phox Interaction in Colitis-Induced Mice
- PMID: 34943057
- PMCID: PMC8750874
- DOI: 10.3390/antiox10121954
Mito-TIPTP Increases Mitochondrial Function by Repressing the Rubicon-p22phox Interaction in Colitis-Induced Mice
Erratum in
-
Correction: Kim et al. Mito-TIPTP Increases Mitochondrial Function by Repressing the Rubicon-p22phox Interaction in Colitis-Induced Mice. Antioxidants 2021, 10, 1954.Antioxidants (Basel). 2023 Jul 13;12(7):1415. doi: 10.3390/antiox12071415. Antioxidants (Basel). 2023. PMID: 37508020 Free PMC article.
Abstract
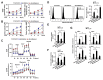
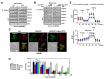
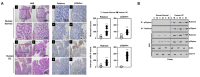
The run/cysteine-rich-domain-containing Beclin1-interacting autophagy protein (Rubicon) is essential for the regulation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase by interacting with p22phox to trigger the production of reactive oxygen species (ROS) in immune cells. In a previous study, we demonstrated that the interaction of Rubicon with p22phox increases cellular ROS levels. The correlation between Rubicon and mitochondrial ROS (mtROS) is poorly understood. Here, we report that Rubicon interacts with p22phox in the outer mitochondrial membrane in macrophages and patients with human ulcerative colitis. Upon lipopolysaccharide (LPS) activation, the binding of Rubicon to p22phox was elevated, and increased not only cellular ROS levels but also mtROS, with an impairment of mitochondrial complex III and mitochondrial biogenesis in macrophages. Furthermore, increased Rubicon decreases mitochondrial metabolic flux in macrophages. Mito-TIPTP, which is a p22phox inhibitor containing a mitochondrial translocation signal, enhances mitochondrial function by inhibiting the association between Rubicon and p22phox in LPS-primed bone-marrow-derived macrophages (BMDMs) treated with adenosine triphosphate (ATP) or dextran sulfate sodium (DSS). Remarkably, Mito-TIPTP exhibited a therapeutic effect by decreasing mtROS in DSS-induced acute or chronic colitis mouse models. Thus, our findings suggest that Mito-TIPTP is a potential therapeutic agent for colitis by inhibiting the interaction between Rubicon and p22phox to recover mitochondrial function.
Keywords: Rubicon; colitis; mitochondria; p22phox; reactive oxygen species.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

