Anthocyanins: Factors Affecting Their Stability and Degradation
- PMID: 34943070
- PMCID: PMC8750456
- DOI: 10.3390/antiox10121967
Anthocyanins: Factors Affecting Their Stability and Degradation
Abstract
Anthocyanins are secondary metabolites and water-soluble pigments belonging to the phenolic group, with important functions in nature such as seed dispersal, pollination and development of plant organs. In addition to these important roles in plant life, anthocyanins are also used as natural pigments in various industries, due to the color palette they can produce from red to blue and purple. In addition, recent research has reported that anthocyanins have important antioxidant, anticancer, anti-inflammatory and antimicrobial properties, which can be used in the chemoprevention of various diseases such as diabetes, obesity and even cancer. However, anthocyanins have a major disadvantage, namely their low stability. Thus, their stability is influenced by a number of factors such as pH, light, temperature, co-pigmentation, sulfites, ascorbic acid, oxygen and enzymes. As such, this review aims at summarizing the effects of these factors on the stability of anthocyanins and their degradation. From this point of view, it is very important to be precisely aware of the impact that each parameter has on the stability of anthocyanins, in order to minimize their negative action and subsequently potentiate their beneficial health effects.
Keywords: anthocyanins; degradation; health benefit; pigments; stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




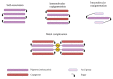

References
-
- Liu P., Li W., Hu Z., Qin X., Liu G. Isolation, purification, identification, and stability of anthocyanins from Lycium ruthenicum Murr. LWT. 2020;126:109334. doi: 10.1016/j.lwt.2020.109334. - DOI
-
- Brooks M.S.-L., Celli G.B. Anthocyanins from Natural Sources: Exploiting Targeted Delivery for Improved Health. Royal Society of Chemistry; Londone, UK: 2019.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

