The Protective Effects of Carrageenan Oligosaccharides on Intestinal Oxidative Stress Damage of Female Drosophila melanogaster
- PMID: 34943099
- PMCID: PMC8698627
- DOI: 10.3390/antiox10121996
The Protective Effects of Carrageenan Oligosaccharides on Intestinal Oxidative Stress Damage of Female Drosophila melanogaster
Abstract
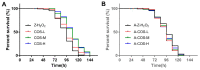
Carrageenan oligosaccharides (COS) have been reported to possess excellent antioxidant activities, but the underlying mechanism remains poorly understood. In this study, H2O2 was applied to trigger oxidative stress. The results showed that the addition of COS could effectively extend the lifespan of female Drosophila, which was associated with improvements by COS on the antioxidant defense system, including a decrease in MDA, the enhanced activities of SOD and CAT, the reduction of ROS in intestinal epithelial cells, and the up-regulation of antioxidant-relevant genes (GCL, GSTs, Nrf2, SOD). Meanwhile, the axenic female Drosophila fed with COS showed almost no improvement in the above measurements after H2O2 treatment, which highlighted the antioxidant mechanism of COS was closely related to intestinal microorganisms. Then, 16S rDNA high-throughput sequencing was applied and the result showed that the addition of COS in diets contributed to the diversity and abundance of intestinal flora in H2O2 induced female Drosophila. Moreover, COS significantly inhibited the expression of gene mTOR, elevated its downstream gene 4E-BP, and further inhibited autophagy-relevant genes (AMPKα, Atg1, Atg5, Atg8a) in H2O2 induced female Drosophila. The inhibition of the mTOR pathway and the activation of autophagy was probably mediated by the antioxidant effects of COS. These results provide potential evidence for further understanding of COS as an intestinal antioxidant.
Keywords: Drosophila melanogaster; carrageenan oligosaccharides; gut flora; intestinal oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








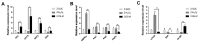
References
-
- Mahajan L., Verma P.K., Raina R., Pankaj N.K., Sood S., Singh M. Alteration in thiols homeostasis, protein and lipid peroxidation in renal tissue following subacute oral exposure of imidacloprid and arsenic in Wistar rats. Toxicol. Rep. 2018;5:1114–1119. doi: 10.1016/j.toxrep.2018.11.003. - DOI - PMC - PubMed
-
- Oke G.O., Abiodun A.A., Imafidon C.E., Monsi B.F. Zingiber officinale (Roscoe) mitigates CCl4-induced liver histopathology and biochemical derangements through antioxidant, membrane-stabilizing and tissue-regenerating potentials. Toxicol. Rep. 2019;6:416–425. doi: 10.1016/j.toxrep.2019.05.001. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

