Sheep β-Defensin 2 Regulates Escherichia coli F17 Resistance via NF-κB and MAPK Signaling Pathways in Ovine Intestinal Epithelial Cells
- PMID: 34943272
- PMCID: PMC8698448
- DOI: 10.3390/biology10121356
Sheep β-Defensin 2 Regulates Escherichia coli F17 Resistance via NF-κB and MAPK Signaling Pathways in Ovine Intestinal Epithelial Cells
Abstract
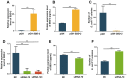
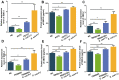
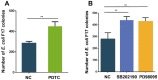
Escherichia coli (E. coli) F17 is a member of enterotoxigenic Escherichia coli, which can cause massive diarrhea and high mortality in newborn lambs. β-defensin is mainly produced by the epithelial tissue of the gastrointestinal tract in response to microbial infection. However, the molecular mechanism of sheep β-defensin 2 (SBD-2) against E. coli F17 remains unclear. This study aims to reveal the antibacterial ability of SBD-2 against E. coli F17 infection in sheep. Firstly, we established the culture system of ovine intestinal epithelial cells (OIECs) in vitro, treated with different concentrations of E. coli F17 for an indicated time. Secondly, we performed RNA interference and overexpression to investigate the effect of SBD-2 expression on E. coli F17 adhesion to OIECs. Finally, inhibitors of NF-κB and MAPK pathways were pre-treated to explore the possible relationship involving in E. coli F17 infection regulating SBD-2 expression. The results showed that E. coli F17 markedly (p < 0.01) upregulated the expression levels of SBD-2 mRNA and protein in a concentration- and time-dependent manner. Overexpression of SBD-2 contributed to enhancing E. coli F17 resistance in OIECs, while silencing SBD-2 dramatically improved the adhesion of E. coli F17 to OIECs (p < 0.05 or p < 0.01). Furthermore, E. coli F17 stimulated SBD-2 expression was obviously decreased by pre-treatment with NF-κB inhibitor PDTC, p38 MAPK inhibitor SB202190 and ERK1/2 MAPK inhibitor PD98095 (p < 0.05 or p < 0.01). Interestingly, adhesion of E. coli F17 to OIECs were highly enhanced by pre-treated with PDTC, SB202190 and PD98095. Our data suggested that SBD-2 could inhibit E. coli F17 infection in OIECs, possibly through NF-κB and MAPK signaling pathways. Our results provide useful theoretical basis on developing anti-infective drug and breeding for E. coli diarrhea disease-resistant sheep.
Keywords: Escherichia coli F17; MAPK pathway; NF-κB pathway; SBD-2; inflammation; sheep.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Shabana I.I., Bouqellah N.A., Zaraket H. Investigation of viral and bacterial enteropathogens of diarrheic sheep and goats in Medina, Saudi Arabia. Trop. Biomed. 2017;34:944–955. - PubMed
-
- Lintermans P.F., Pohl P., Bertels A., Charlier G., Vandekerckhove J., Van Damme J., Schoup J., Schlicker C., Korhonen T., De Greve H., et al. Characterization and purification of the F17 adhesin on the surface of bovine enteropathogenic and septicemic Escherichia coli. Am. J. Vet. Res. 1988;49:1794–1799. - PubMed
Grants and funding
- 32061143036/National Natural Science Foundation of China-CGIAR
- 31872333, 32172689/National Natural Science Foundation of China
- BE2018354/Key Research and Development Plan (modern agriculture) in Jiangsu Province
- PZCZ201739/Major New Varieties of Agricultural Projects in Jiangsu Province
- CX(18)2003/Jiangsu Agricultural Science and Technology Innovation Fund
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

