Gas6/TAM Signalling Negatively Regulates Inflammatory Induction of GM-CSF in Mouse Brain Microglia
- PMID: 34943789
- PMCID: PMC8699038
- DOI: 10.3390/cells10123281
Gas6/TAM Signalling Negatively Regulates Inflammatory Induction of GM-CSF in Mouse Brain Microglia
Abstract
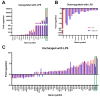
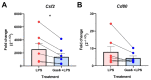
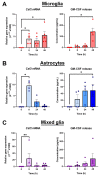
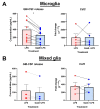
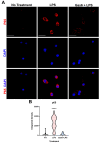
Microglia and astrocytes are the main CNS glial cells responsible for the neuroinflammatory response, where they release a plethora of cytokines into the CNS inflammatory milieu. The TAM (Tyro3, Axl, Mer) receptors and their main ligand Gas6 are regulators of this response, however, the underlying mechanisms remain to be determined. We investigated the ability of Gas6 to modulate the CNS glial inflammatory response to lipopolysaccharide (LPS), a strong pro-inflammatory agent, through a qPCR array that explored Toll-like receptor signalling pathway-associated genes in primary cultured mouse microglia. We identified the Csf2 gene, encoding granulocyte-macrophage colony-stimulating factor (GM-CSF), as a major Gas6 target gene whose induction by LPS was markedly blunted by Gas6. Both the Csf2 gene induction and the suppressive effect of Gas6 on this were emulated through measurement of GM-CSF protein release by cells. We found distinct profiles of GM-CSF induction in different glial cell types, with microglia being most responsive during inflammation. Also, Gas6 markedly inhibited the LPS-stimulated nuclear translocation of NF-κB p65 protein in microglia. These results illustrate microglia as a major resident CNS cellular source of GM-CSF as part of the neuroinflammatory response, and that Gas6/TAM signalling inhibits this response through suppression of NF-κB signalling.
Keywords: GM-CSF; Gas6; NF-κB; TAM receptors; astrocytes; glial cells; lipopolysaccharide; microglia; neuroinflammation; primary culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

