Microvalve Bioprinting of MSC-Chondrocyte Co-Cultures
- PMID: 34943837
- PMCID: PMC8699323
- DOI: 10.3390/cells10123329
Microvalve Bioprinting of MSC-Chondrocyte Co-Cultures
Abstract
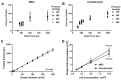
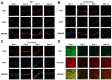
Recent improvements within the fields of high-throughput screening and 3D tissue culture have provided the possibility of developing in vitro micro-tissue models that can be used to study diseases and screen potential new therapies. This paper reports a proof-of-concept study on the use of microvalve-based bioprinting to create laminar MSC-chondrocyte co-cultures to investigate whether the use of MSCs in ACI procedures would stimulate enhanced ECM production by chondrocytes. Microvalve-based bioprinting uses small-scale solenoid valves (microvalves) to deposit cells suspended in media in a consistent and repeatable manner. In this case, MSCs and chondrocytes have been sequentially printed into an insert-based transwell system in order to create a laminar co-culture, with variations in the ratios of the cell types used to investigate the potential for MSCs to stimulate ECM production. Histological and indirect immunofluorescence staining revealed the formation of dense tissue structures within the chondrocyte and MSC-chondrocyte cell co-cultures, alongside the establishment of a proliferative region at the base of the tissue. No stimulatory or inhibitory effect in terms of ECM production was observed through the introduction of MSCs, although the potential for an immunomodulatory benefit remains. This study, therefore, provides a novel method to enable the scalable production of therapeutically relevant micro-tissue models that can be used for in vitro research to optimise ACI procedures.
Keywords: 3D cell culture; autologous chondrocyte implantation; biofabrication; bioprinting; drop-on-demand; micro-tissue; microvalve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
-
- National Institute for Health and Care Excellence . Osteoarthritis: Care and Management—Clinical Guideline CG177. National Institute for Health and Care Excellence; London, UK: 2014. [(accessed on 26 November 2021)]. Available online: https://www.nice.org.uk/guidance/cg177.
-
- Richardson J.B., Wright K.T., Wales J., Kuiper J.H., McCarthy H., Gallacher P., Harrison P.E., Roberts S. Efficacy and safety of autologous cell therapies for knee cartilage defects (autologous stem cells, chondrocytes or the two): Randomized controlled trial design. Regen. Med. 2017;12:493–501. doi: 10.2217/rme-2017-0032. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

