Molecular Signaling to Preserve Mitochondrial Integrity against Ischemic Stress in the Heart: Rescue or Remove Mitochondria in Danger
- PMID: 34943839
- PMCID: PMC8699551
- DOI: 10.3390/cells10123330
Molecular Signaling to Preserve Mitochondrial Integrity against Ischemic Stress in the Heart: Rescue or Remove Mitochondria in Danger
Abstract
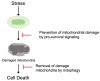
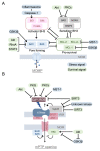
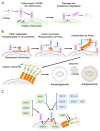
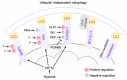
Cardiovascular diseases are one of the leading causes of death and global health problems worldwide, and ischemic heart disease is the most common cause of heart failure (HF). The heart is a high-energy demanding organ, and myocardial energy reserves are limited. Mitochondria are the powerhouses of the cell, but under stress conditions, they become damaged, release necrotic and apoptotic factors, and contribute to cell death. Loss of cardiomyocytes plays a significant role in ischemic heart disease. In response to stress, protective signaling pathways are activated to limit mitochondrial deterioration and protect the heart. To prevent mitochondrial death pathways, damaged mitochondria are removed by mitochondrial autophagy (mitophagy). Mitochondrial quality control mediated by mitophagy is functionally linked to mitochondrial dynamics. This review provides a current understanding of the signaling mechanisms by which the integrity of mitochondria is preserved in the heart against ischemic stress.
Keywords: BCL-2 family proteins; cell death; heart; ischemia; mPTP; mitochondria; mitophagy; pro-survival signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. - DOI - PubMed
-
- Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

