Pluripotent Stem Cells for Spinal Cord Injury Repair
- PMID: 34943842
- PMCID: PMC8699436
- DOI: 10.3390/cells10123334
Pluripotent Stem Cells for Spinal Cord Injury Repair
Abstract
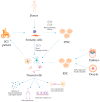
Spinal cord injury (SCI) is a devastating condition of the central nervous system that strongly reduces the patient's quality of life and has large financial costs for the healthcare system. Cell therapy has shown considerable therapeutic potential for SCI treatment in different animal models. Although many different cell types have been investigated with the goal of promoting repair and recovery from injury, stem cells appear to be the most promising. Here, we review the experimental approaches that have been carried out with pluripotent stem cells, a cell type that, due to its inherent plasticity, self-renewal, and differentiation potential, represents an attractive source for the development of new cell therapies for SCI. We will focus on several key observations that illustrate the potential of cell therapy for SCI, and we will attempt to draw some conclusions from the studies performed to date.
Keywords: ESC; NSC; PSC; animal models; cell therapy; iPSC; tetraplegia.
Conflict of interest statement
B.F.-M. is the author of a patent application for the use of cerebrospinal fluid-derived NSC (International Publication Number WO 2019/229096). The other authors indicated no potential conflict of interest.
Figures

References
-
- Takami T., Oudega M., Bates M.L., Wood P.M., Kleitman N., Bunge M.B. Schwann Cell But Not Olfactory Ensheathing Glia Transplants Improve Hindlimb Locomotor Performance in the Moderately Contused Adult Rat Thoracic Spinal Cord. J. Neurosci. 2002;22:6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

