Tissue-Nonspecific Alkaline Phosphatase, a Possible Mediator of Cell Maturation: Towards a New Paradigm
- PMID: 34943845
- PMCID: PMC8699127
- DOI: 10.3390/cells10123338
Tissue-Nonspecific Alkaline Phosphatase, a Possible Mediator of Cell Maturation: Towards a New Paradigm
Abstract
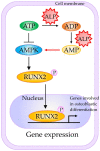
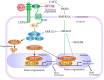
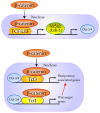
Alkaline phosphatase (ALP) is a ubiquitous membrane-bound glycoprotein capable of providing inorganic phosphate by catalyzing the hydrolysis of organic phosphate esters, or removing inorganic pyrophosphate that inhibits calcification. In humans, four forms of ALP cDNA have been cloned, among which tissue-nonspecific ALP (TNSALP) (TNSALP) is widely distributed in the liver, bone, and kidney, making it an important marker in clinical and basic research. Interestingly, TNSALP is highly expressed in juvenile cells, such as pluripotent stem cells (i.e., embryonic stem cells and induced pluripotent stem cells (iPSCs)) and somatic stem cells (i.e., neuronal stem cells and bone marrow mesenchymal stem cells). Hypophosphatasia is a genetic disorder causing defects in bone and tooth development as well as neurogenesis. Mutations in the gene coding for TNSALP are thought to be responsible for the abnormalities, suggesting the essential role of TNSALP in these events. Moreover, a reverse-genetics-based study using mice revealed that TNSALP is important in bone and tooth development as well as neurogenesis. However, little is known about the role of TNSALP in the maintenance and differentiation of juvenile cells. Recently, it was reported that cells enriched with TNSALP are more easily reprogrammed into iPSCs than those with less TNSALP. Furthermore, in bone marrow stem cells, ALP could function as a "signal regulator" deciding the fate of these cells. In this review, we summarize the properties of ALP and the background of ALP gene analysis and its manipulation, with a special focus on the potential role of TNSALP in the generation (and possibly maintenance) of juvenile cells.
Keywords: alkaline phosphatase; induced pluripotent stem cells; juvenile cells; pluripotent stem cells; reprogramming; signal regulator; somatic stem cells; tissue-nonspecific alkaline phosphatase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

