Geniposide Ameliorated Dexamethasone-Induced Cholesterol Accumulation in Osteoblasts by Mediating the GLP-1R/ABCA1 Axis
- PMID: 34943934
- PMCID: PMC8699812
- DOI: 10.3390/cells10123424
Geniposide Ameliorated Dexamethasone-Induced Cholesterol Accumulation in Osteoblasts by Mediating the GLP-1R/ABCA1 Axis
Erratum in
-
Correction: Zheng et al. Geniposide Ameliorated Dexamethasone-Induced Cholesterol Accumulation in Osteoblasts by Mediating the GLP-1R/ABCA1 Axis. Cells 2021, 10, 3424.Cells. 2022 Nov 30;11(23):3838. doi: 10.3390/cells11233838. Cells. 2022. PMID: 36497203 Free PMC article.
Abstract
Background: Overexposure to glucocorticoid (GC) produces various clinical complications, including osteoporosis (OP), dyslipidemia, and hypercholesterolemia. Geniposide (GEN) is a natural iridoid compound isolated from Eucommia ulmoides. Our previous study found that GEN could alleviate dexamethasone (DEX)-induced differentiation inhibition of MC3T3-E1 cells. However, whether GEN protected against Dex-induced cholesterol accumulation in osteoblasts was still unclear.
Methods: DEX was used to induce rat OP. Micro-CT data was obtained. The ALP activity and mineralization were determined by the staining assays, and the total intracellular cholesterol was determined by the ELISA kits. The protein expression was detected by western blot.
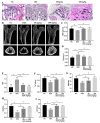
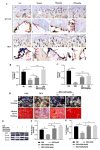
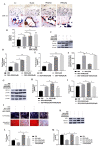
Results: GEN ameliorated Dex-induced micro-structure damages and cell differentiation inhibition in the bone trabecula in rats. In MC3T3-E1 cells, Dex enhanced the total intracellular cholesterol, which reduced the activity of cell proliferation and differentiation. Effectively, GEN decreased DEX-induced cholesterol accumulation, enhanced cell differentiation, and upregulated the expression of the GLP-1R/ABCA1 axis. In addition, inhibition of ABAC1 expression reversed the actions of GEN. Treatment with Exendin9-39, a GLP-1R inhibitor, could abrogate the protective activity of GEN.
Conclusions: GEN ameliorated Dex-induced accumulation of cholesterol and inhibition of cell differentiation by mediating the GLP-1R/ABCA1 axis in MC3T3-E1 cells.
Keywords: ABCA1; GLP-1R; cholesterol; geniposide; glucocorticoid; osteoporosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Geniposide ameliorates cholesterol accumulation and promotes osteoblast differentiation by mediating the GLP-1R/AMPK/SREBP2 pathway.J Orthop Surg Res. 2025 May 26;20(1):514. doi: 10.1186/s13018-025-05945-3. J Orthop Surg Res. 2025. PMID: 40414881 Free PMC article.
-
Geniposide alleviates cholesterol-induced endoplasmic reticulum stress and apoptosis in osteoblasts by mediating the GLP-1R/ABCA1 pathway.J Orthop Surg Res. 2024 Mar 11;19(1):179. doi: 10.1186/s13018-024-04665-4. J Orthop Surg Res. 2024. PMID: 38468352 Free PMC article.
-
Geniposide ameliorated dexamethasone-induced endoplasmic reticulum stress and mitochondrial apoptosis in osteoblasts.J Ethnopharmacol. 2022 Jun 12;291:115154. doi: 10.1016/j.jep.2022.115154. Epub 2022 Feb 28. J Ethnopharmacol. 2022. PMID: 35240241
-
Geniposide ameliorates glucocorticoid-induced osteoblast apoptosis by activating autophagy.Biomed Pharmacother. 2022 Nov;155:113829. doi: 10.1016/j.biopha.2022.113829. Epub 2022 Oct 7. Biomed Pharmacother. 2022. PMID: 36271582
-
Eucommia ulmoides Oliver polysaccharide alleviates glucocorticoid-induced osteoporosis by stimulating bone formation via ERK/BMP-2/SMAD signaling.Sci Rep. 2024 Nov 28;14(1):29647. doi: 10.1038/s41598-024-80859-4. Sci Rep. 2024. PMID: 39609585 Free PMC article.
Cited by
-
Pathogenic mechanisms of glucocorticoid-induced osteoporosis.Cytokine Growth Factor Rev. 2023 Apr;70:54-66. doi: 10.1016/j.cytogfr.2023.03.002. Epub 2023 Mar 5. Cytokine Growth Factor Rev. 2023. PMID: 36906448 Free PMC article. Review.
-
The Beneficial Effects of Geniposide on Glucose and Lipid Metabolism: A Review.Drug Des Devel Ther. 2022 Sep 30;16:3365-3383. doi: 10.2147/DDDT.S378976. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36213380 Free PMC article. Review.
-
The protective activity of genistein against bone and cartilage diseases.Front Pharmacol. 2022 Sep 8;13:1016981. doi: 10.3389/fphar.2022.1016981. eCollection 2022. Front Pharmacol. 2022. PMID: 36160403 Free PMC article. Review.
-
Macrophage-derived extracellular vesicles regulate skeletal stem/progenitor Cell lineage fate and bone deterioration in obesity.Bioact Mater. 2024 Jul 4;36:508-523. doi: 10.1016/j.bioactmat.2024.06.035. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 39072285 Free PMC article.
-
Cinchonine, a Potential Oral Small-Molecule Glucagon-Like Peptide-1 Receptor Agonist, Lowers Blood Glucose and Ameliorates Non-Alcoholic Steatohepatitis.Drug Des Devel Ther. 2023 May 11;17:1417-1432. doi: 10.2147/DDDT.S404055. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37197367 Free PMC article.
References
-
- Hayashi K., Yamaguchi T., Yano S., Kanazawa I., Yamauchi M., Yamamoto M., Sugimoto T. BMP/Wnt antagonists are upregulated by dexamethasone in osteoblasts and reversed by alendronate and PTH: Potential therapeutic targets for glucocorticoid-induced osteoporosis. Biochem. Biophys. Res. Commun. 2009;379:261–266. doi: 10.1016/j.bbrc.2008.12.035. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82060407, 81860261, 81860388, and 81960883/National Natural Science Foundation of China
- 20202BABL206122 and 20202BABL206134/the Natural Science Foundation of Jiangxi Province
- GJJ201501 and GJJ201510/Scientific Research Fund of Jiangxi Provincial Education Department
- TD201707/Innovative Teamwork Project of Gannan Medical University
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

