Homeostatic Regulation of Glucocorticoid Receptor Activity by Hypoxia-Inducible Factor 1: From Physiology to Clinic
- PMID: 34943949
- PMCID: PMC8699886
- DOI: 10.3390/cells10123441
Homeostatic Regulation of Glucocorticoid Receptor Activity by Hypoxia-Inducible Factor 1: From Physiology to Clinic
Abstract
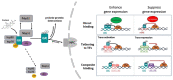
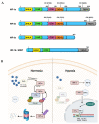
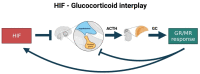
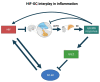
Glucocorticoids (GCs) represent a well-known class of lipophilic steroid hormones biosynthesised, with a circadian rhythm, by the adrenal glands in humans and by the inter-renal tissue in teleost fish (e.g., zebrafish). GCs play a key role in the regulation of numerous physiological processes, including inflammation, glucose, lipid, protein metabolism and stress response. This is achieved through binding to their cognate receptor, GR, which functions as a ligand-activated transcription factor. Due to their potent anti-inflammatory and immune-suppressive action, synthetic GCs are broadly used for treating pathological disorders that are very often linked to hypoxia (e.g., rheumatoid arthritis, inflammatory, allergic, infectious, and autoimmune diseases, among others) as well as to prevent graft rejections and against immune system malignancies. However, due to the presence of adverse effects and GC resistance their therapeutic benefits are limited in patients chronically treated with steroids. For this reason, understanding how to fine-tune GR activity is crucial in the search for novel therapeutic strategies aimed at reducing GC-related side effects and effectively restoring homeostasis. Recent research has uncovered novel mechanisms that inhibit GR function, thereby causing glucocorticoid resistance, and has produced some surprising new findings. In this review we analyse these mechanisms and focus on the crosstalk between GR and HIF signalling. Indeed, its comprehension may provide new routes to develop novel therapeutic targets for effectively treating immune and inflammatory response and to simultaneously facilitate the development of innovative GCs with a better benefits-risk ratio.
Keywords: crosstalk; glucocorticoid; glucocorticoid receptor; hypoxia inducible factor; immune modulations; inflammation.
Conflict of interest statement
Authors report no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

