Senescence Alterations in Pulmonary Hypertension
- PMID: 34943963
- PMCID: PMC8700581
- DOI: 10.3390/cells10123456
Senescence Alterations in Pulmonary Hypertension
Abstract
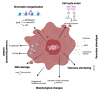
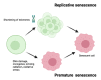
Cellular senescence is the arrest of normal cell division and is commonly associated with aging. The interest in the role of cellular senescence in lung diseases derives from the observation of markers of senescence in chronic obstructive pulmonary disease (COPD), pulmonary fibrosis (IPF), and pulmonary hypertension (PH). Accumulation of senescent cells and the senescence-associated secretory phenotype in the lung of aged patients may lead to mild persistent inflammation, which results in tissue damage. Oxidative stress due to environmental exposures such as cigarette smoke also promotes cellular senescence, together with additional forms of cellular stress such as mitochondrial dysfunction and endoplasmic reticulum stress. Growing recent evidence indicate that senescent cell phenotypes are observed in pulmonary artery smooth muscle cells and endothelial cells of patients with PH, contributing to pulmonary artery remodeling and PH development. In this review, we analyze the role of different senescence cell phenotypes contributing to the pulmonary artery remodeling process in different PH clinical entities. Different molecular pathway activation and cellular functions derived from senescence activation will be analyzed and discussed as promising targets to develop future senotherapies as promising treatments to attenuate pulmonary artery remodeling in PH.
Keywords: SASP; pulmonary hypertension; senescence; senolytics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
p16-3MR: A Novel Model to Study Cellular Senescence in Cigarette Smoke-Induced Lung Injuries.Int J Mol Sci. 2021 May 3;22(9):4834. doi: 10.3390/ijms22094834. Int J Mol Sci. 2021. PMID: 34063608 Free PMC article.
-
Senotherapy for lung diseases.Adv Pharmacol. 2023;98:249-271. doi: 10.1016/bs.apha.2023.04.001. Epub 2023 Apr 20. Adv Pharmacol. 2023. PMID: 37524489
-
Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease.Circ Res. 2011 Aug 19;109(5):543-53. doi: 10.1161/CIRCRESAHA.111.241299. Epub 2011 Jun 30. Circ Res. 2011. PMID: 21719760 Free PMC article.
-
Cellular Senescence in Aging Lungs and Diseases.Cells. 2022 May 29;11(11):1781. doi: 10.3390/cells11111781. Cells. 2022. PMID: 35681476 Free PMC article. Review.
-
Senescence: Pathogenic Driver in Chronic Obstructive Pulmonary Disease.Medicina (Kaunas). 2022 Jun 17;58(6):817. doi: 10.3390/medicina58060817. Medicina (Kaunas). 2022. PMID: 35744080 Free PMC article. Review.
Cited by
-
Identification and experimental verification of senescence-related gene signatures and molecular subtypes in idiopathic pulmonary arterial hypertension.Sci Rep. 2024 Sep 27;14(1):22157. doi: 10.1038/s41598-024-72979-8. Sci Rep. 2024. PMID: 39333589 Free PMC article.
-
Unraveling the role of HIF and epigenetic regulation in pulmonary arterial hypertension: implications for clinical research and its therapeutic approach.Front Med (Lausanne). 2024 Oct 10;11:1460376. doi: 10.3389/fmed.2024.1460376. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39450110 Free PMC article. Review.
-
Acid ceramidase gene therapy ameliorates pulmonary arterial hypertension with right heart dysfunction.Respir Res. 2023 Aug 11;24(1):197. doi: 10.1186/s12931-023-02487-2. Respir Res. 2023. PMID: 37568148 Free PMC article.
-
A Unified Model of Age-Related Cardiovascular Disease.Biology (Basel). 2022 Dec 6;11(12):1768. doi: 10.3390/biology11121768. Biology (Basel). 2022. PMID: 36552277 Free PMC article. Review.
-
Modelling of biological age in stable and acute exacerbations of chronic obstructive pulmonary disease.BMC Pulm Med. 2025 Aug 19;25(1):398. doi: 10.1186/s12890-025-03841-4. BMC Pulm Med. 2025. PMID: 40830456 Free PMC article.
References
-
- Contrepois K., Coudereau C., Benayoun B.A., Schuler N., Roux P.-F., Bischof O., Courbeyrette R., Carvalho C., Thuret J.-Y., Ma Z., et al. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat. Commun. 2017;8:14995. doi: 10.1038/ncomms14995. - DOI - PMC - PubMed
-
- Coppé J.-P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J.N., Nelson P.S., Desprez P.-Y., Campisi J. Senescence-associated secretory Phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

