Alternative Splicing of MAPKs in the Regulation of Signaling Specificity
- PMID: 34943973
- PMCID: PMC8699841
- DOI: 10.3390/cells10123466
Alternative Splicing of MAPKs in the Regulation of Signaling Specificity
Abstract
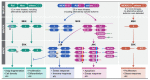
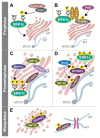
The mitogen-activated protein kinase (MAPK) cascades transmit signals from extracellular stimuli to a variety of distinct cellular processes. The MAPKKs in each cascade specifically phosphorylate and activate their cognate MAPKs, indicating that this step funnels various signals into a seemingly linear pathway. Still, the effects of these cascades vary significantly, depending on the identity of the extracellular signals, which gives rise to proper outcomes. Therefore, it is clear that the specificity of the signals transmitted through the cascades is tightly regulated in order to secure the desired cell fate. Indeed, many regulatory components or processes that extend the specificity of the cascades have been identified. Here, we focus on a less discussed mechanism, that is, the role of distinct components in each tier of the cascade in extending the signaling specificity. We cover the role of distinct genes, and the alternatively spliced isoforms of MAPKKs and MAPKs, in the signaling specificity. The alternatively spliced MEK1b and ERK1c, which form an independent signaling route, are used as the main example. Unlike MEK1/2 and ERK1/2, this route's functions are limited, including mainly the regulation of mitotic Golgi fragmentation. The unique roles of the alternatively spliced isoforms indicate that these components play an essential role in determining the proper cell fate in response to distinct stimulations.
Keywords: ERK; ERK1c; JNK; MAPK; alternative splicing; p38.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Specific phosphorylation and activation of ERK1c by MEK1b: a unique route in the ERK cascade.Genes Dev. 2009 Aug 1;23(15):1779-90. doi: 10.1101/gad.523909. Genes Dev. 2009. PMID: 19651986 Free PMC article.
-
ERK1c regulates Golgi fragmentation during mitosis.J Cell Biol. 2006 Mar 13;172(6):885-97. doi: 10.1083/jcb.200509063. J Cell Biol. 2006. PMID: 16533948 Free PMC article.
-
The MAP kinase signaling cascades: a system of hundreds of components regulates a diverse array of physiological functions.Methods Mol Biol. 2010;661:3-38. doi: 10.1007/978-1-60761-795-2_1. Methods Mol Biol. 2010. PMID: 20811974 Review.
-
Extracellular signal-regulated kinase 1c (ERK1c), a novel 42-kilodalton ERK, demonstrates unique modes of regulation, localization, and function.Mol Cell Biol. 2004 Nov;24(22):10000-15. doi: 10.1128/MCB.24.22.10000-10015.2004. Mol Cell Biol. 2004. PMID: 15509801 Free PMC article.
-
Role of MAPKs in development and differentiation: lessons from knockout mice.Biochimie. 2006 Sep;88(9):1091-8. doi: 10.1016/j.biochi.2006.06.003. Epub 2006 Jun 27. Biochimie. 2006. PMID: 16854512 Review.
Cited by
-
Network Pharmacology and Experimental Verification Revealed the Mechanism of Yiqi Jianpi Recipe on Chronic Obstructive Pulmonary Disease.Evid Based Complement Alternat Med. 2022 Sep 7;2022:8823231. doi: 10.1155/2022/8823231. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36118092 Free PMC article.
-
ERK1b, a 46-kDa ERK isoform that is differentially regulated by MEK.Cell Biol Int. 2022 Jul;46(7):1021-1035. doi: 10.1002/cbin.11801. Epub 2022 Apr 4. Cell Biol Int. 2022. PMID: 35332606 Free PMC article.
-
GLUT2 regulation of p38 MAPK isoform protein expression and p38 phosphorylation in male versus female rat hypothalamic primary astrocyte Cultures.IBRO Neurosci Rep. 2024 May 22;16:635-642. doi: 10.1016/j.ibneur.2024.05.008. eCollection 2024 Jun. IBRO Neurosci Rep. 2024. PMID: 38832087 Free PMC article.
-
Network Pharmacology-Based Strategy for Predicting Therapy Targets of Citri Reticulatae Pericarpium on Myocardial Hypertrophy.Biomed Res Int. 2022 Mar 2;2022:4293265. doi: 10.1155/2022/4293265. eCollection 2022. Biomed Res Int. 2022. PMID: 35281609 Free PMC article.
-
Special Issue: MAPK Signaling Cascades in Human Health and Diseases.Int J Mol Sci. 2024 Oct 18;25(20):11226. doi: 10.3390/ijms252011226. Int J Mol Sci. 2024. PMID: 39457006 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

