Long-Term Treatment with Atypical Antipsychotic Iloperidone Modulates Cytochrome P450 2D (CYP2D) Expression and Activity in the Liver and Brain via Different Mechanisms
- PMID: 34943983
- PMCID: PMC8700221
- DOI: 10.3390/cells10123472
Long-Term Treatment with Atypical Antipsychotic Iloperidone Modulates Cytochrome P450 2D (CYP2D) Expression and Activity in the Liver and Brain via Different Mechanisms
Erratum in
-
Correction: Danek, P.J.; Daniel, W.A. Long-Term Treatment with Atypical Antipsychotic Iloperidone Modulates Cytochrome P450 2D (CYP2D) Expression and Activity in the Liver and Brain via Different Mechanisms. Cells 2021, 10, 3472.Cells. 2024 Dec 23;13(24):2133. doi: 10.3390/cells13242133. Cells. 2024. PMID: 39768231 Free PMC article.
Abstract
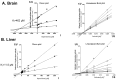
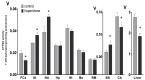
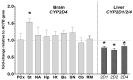
CYP2D enzymes engage in the synthesis of endogenous neuroactive substances (dopamine, serotonin) and in the metabolism of neurosteroids. The present work investigates the effect of iloperidone on CYP2D enzyme expression and activity in rat brains and livers. Iloperidone exerted a weak direct inhibitory effect on CYP2D activity in vitro in the liver and brain microsomes (Ki = 11.5 μM and Ki = 462 μM, respectively). However, a two-week treatment with iloperidone (1 mg/kg ip.) produced a significant decrease in the activity of liver CYP2D, which correlated positively with the reduced CYP2D1, CYP2D2 and CYP2D4 protein and mRNA levels. Like in the liver, iloperidone reduced CYP2D activity and protein levels in the frontal cortex and cerebellum but enhanced these levels in the nucleus accumbens, striatum and substantia nigra. Chronic iloperidone did not change the brain CYP2D4 mRNA levels, except in the striatum, where they were significantly increased. In conclusion, by affecting CYP2D activity in the brain, iloperidone may modify its pharmacological effect, via influencing the rate of dopamine and serotonin synthesis or the metabolism of neurosteroids. By elevating the CYP2D expression/activity in the substantia nigra and striatum (i.e., in the dopaminergic nigrostriatal pathway), iloperidone may attenuate extrapyramidal symptoms, while by decreasing the CYP2D activity and metabolism of neurosteroiods in the frontal cortex and cerebellum, iloperidone can have beneficial effects in the treatment of schizophrenia. In the liver, pharmacokinetic interactions involving chronic iloperidone and CYP2D substrates are likely to occur.
Keywords: brain; cytochrome P450 2D expression/activity; iloperidone; liver; prolonged administration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

