Cannabidiol Inhibits Tau Aggregation In Vitro
- PMID: 34944028
- PMCID: PMC8700709
- DOI: 10.3390/cells10123521
Cannabidiol Inhibits Tau Aggregation In Vitro
Abstract
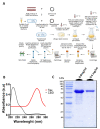
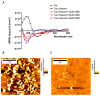
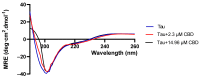
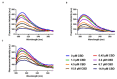
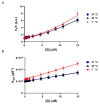
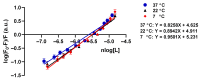
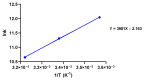
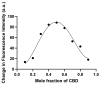
A hallmark of Alzheimer's disease (AD) is the accumulation of tau protein in the brain. Compelling evidence indicates that the presence of tau aggregates causes irreversible neuronal destruction, eventually leading to synaptic loss. So far, the inhibition of tau aggregation has been recognized as one of the most effective therapeutic strategies. Cannabidiol (CBD), a major component found in Cannabis sativa L., has antioxidant activities as well as numerous neuroprotective features. Therefore, we hypothesize that CBD may serve as a potent substance to hamper tau aggregation in AD. In this study, we aim to investigate the CBD effect on the aggregation of recombinant human tau protein 1N/4R isoform using biochemical methods in vitro and in silico. Using Thioflavin T (ThT) assay, circular dichroism (CD), and atomic force microscopy (AFM), we demonstrated that CBD can suppress tau fibrils formation. Moreover, by quenching assay, docking, and job's plot, we further demonstrated that one molecule of CBD interacts with one molecule of tau protein through a spontaneous binding. Experiments performed by quenching assay, docking, and Thioflavin T assay further established that the main forces are hydrogen Van der Waals and some non-negligible hydrophobic forces, affecting the lag phase of tau protein kinetics. Taken together, this study provides new insights about a natural substance, CBD, for tau therapy which may offer new hope for the treatment of AD.
Keywords: Alzheimer’s disease; amyloid fibrils; cannabidiol; tau protein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- De Anda-Hernández M.A., Lira-De León K.I., Mena R., Campos-Peña V., Meraz-Ríos M.A. Neuroscience–Dealing with Frontiers. IntechOpen; London, UK: 2012. Tau and Amyloid-β Conformational Change to β-Sheet Structures as Effectors in the Development of Alzheimer’s Disease; pp. 363–388.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

