Role of Mitochondrial Protein Import in Age-Related Neurodegenerative and Cardiovascular Diseases
- PMID: 34944035
- PMCID: PMC8699856
- DOI: 10.3390/cells10123528
Role of Mitochondrial Protein Import in Age-Related Neurodegenerative and Cardiovascular Diseases
Abstract
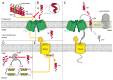
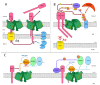
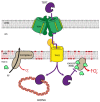
Mitochondria play a critical role in providing energy, maintaining cellular metabolism, and regulating cell survival and death. To carry out these crucial functions, mitochondria employ more than 1500 proteins, distributed between two membranes and two aqueous compartments. An extensive network of dedicated proteins is engaged in importing and sorting these nuclear-encoded proteins into their designated mitochondrial compartments. Defects in this fundamental system are related to a variety of pathologies, particularly engaging the most energy-demanding tissues. In this review, we summarize the state-of-the-art knowledge about the mitochondrial protein import machinery and describe the known interrelation of its failure with age-related neurodegenerative and cardiovascular diseases.
Keywords: Alzheimer’s disease; Parkinson’s disease; TERT; age-related diseases; cardiolipin; cardiovascular disease; mitochondria; mitochondrial protein import.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

