Neutrophil-Derived Extracellular Vesicles Activate Platelets after Pneumolysin Exposure
- PMID: 34944089
- PMCID: PMC8700313
- DOI: 10.3390/cells10123581
Neutrophil-Derived Extracellular Vesicles Activate Platelets after Pneumolysin Exposure
Abstract
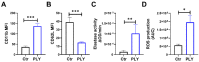
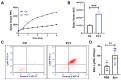
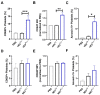
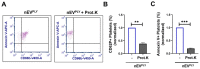
Pneumolysin (PLY) is a pore-forming toxin of Streptococcus pneumoniae that contributes substantially to the inflammatory processes underlying pneumococcal pneumonia and lung injury. Host responses against S. pneumoniae are regulated in part by neutrophils and platelets, both individually and in cooperative interaction. Previous studies have shown that PLY can target both neutrophils and platelets, however, the mechanisms by which PLY directly affects these cells and alters their interactions are not completely understood. In this study, we characterize the effects of PLY on neutrophils and platelets and explore the mechanisms by which PLY may induce neutrophil-platelet interactions. In vitro studies demonstrated that PLY causes the formation of neutrophil extracellular traps (NETs) and the release of extracellular vesicles (EVs) from both human and murine neutrophils. In vivo, neutrophil EV (nEV) levels were increased in mice infected with S. pneumoniae. In platelets, treatment with PLY induced the cell surface expression of P-selectin (CD62P) and binding to annexin V and caused a significant release of platelet EVs (pl-EVs). Moreover, PLY-induced nEVs but not NETs promoted platelet activation. The pretreatment of nEVs with proteinase K inhibited platelet activation, indicating that the surface proteins of nEVs play a role in this process. Our findings demonstrate that PLY activates neutrophils and platelets to release EVs and support an important role for neutrophil EVs in modulating platelet functions in pneumococcal infections.
Keywords: NETs; Streptococcus pneumoniae; lung injury; microvesicles; pneumonia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

