Early Life Inflammation and the Developing Hematopoietic and Immune Systems: The Cochlea as a Sensitive Indicator of Disruption
- PMID: 34944105
- PMCID: PMC8700005
- DOI: 10.3390/cells10123596
Early Life Inflammation and the Developing Hematopoietic and Immune Systems: The Cochlea as a Sensitive Indicator of Disruption
Abstract
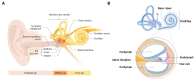
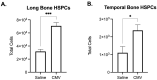
Emerging evidence indicates that perinatal infection and inflammation can influence the developing immune system and may ultimately affect long-term health and disease outcomes in offspring by perturbing tissue and immune homeostasis. We posit that perinatal inflammation influences immune outcomes in offspring by perturbing (1) the development and function of fetal-derived immune cells that regulate tissue development and homeostasis, and (2) the establishment and function of developing hematopoietic stem cells (HSCs) that continually generate immune cells across the lifespan. To disentangle the complexities of these interlinked systems, we propose the cochlea as an ideal model tissue to investigate how perinatal infection affects immune, tissue, and stem cell development. The cochlea contains complex tissue architecture and a rich immune milieu that is established during early life. A wide range of congenital infections cause cochlea dysfunction and sensorineural hearing loss (SNHL), likely attributable to early life inflammation. Furthermore, we show that both immune cells and bone marrow hematopoietic progenitors can be simultaneously analyzed within neonatal cochlear samples. Future work investigating the pathogenesis of SNHL in the context of congenital infection will therefore provide critical information on how perinatal inflammation drives disease susceptibility in offspring.
Keywords: cochlea; congenital infection; cytomegalovirus; fetal-derived immune cells; hematopoiesis; hematopoietic stem and progenitor cells; inflammation; sensorineural hearing loss.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Virus-induced cochlear inflammation in newborn mice alters auditory function.JCI Insight. 2019 Sep 5;4(17):e128878. doi: 10.1172/jci.insight.128878. JCI Insight. 2019. PMID: 31484824 Free PMC article.
-
The role of gene mutations and immune responses in sensorineural hearing loss.Int Immunopharmacol. 2024 Dec 25;143(Pt 3):113515. doi: 10.1016/j.intimp.2024.113515. Epub 2024 Oct 31. Int Immunopharmacol. 2024. PMID: 39486181 Review.
-
Development of the stria vascularis and potassium regulation in the human fetal cochlea: Insights into hereditary sensorineural hearing loss.Dev Neurobiol. 2015 Nov;75(11):1219-40. doi: 10.1002/dneu.22279. Epub 2015 Feb 28. Dev Neurobiol. 2015. PMID: 25663387 Free PMC article.
-
The Severity of Infection Determines the Localization of Damage and Extent of Sensorineural Hearing Loss in Experimental Pneumococcal Meningitis.J Neurosci. 2016 Jul 20;36(29):7740-9. doi: 10.1523/JNEUROSCI.0554-16.2016. J Neurosci. 2016. PMID: 27445150 Free PMC article.
-
[Inflammation and early hematopoiesis].Rinsho Ketsueki. 2018;59(10):1955-1961. doi: 10.11406/rinketsu.59.1955. Rinsho Ketsueki. 2018. PMID: 30305497 Review. Japanese.
Cited by
-
Code Red in the Supply Center: The Impact of Immune Activation on Hematopoiesis.Cells. 2022 May 9;11(9):1586. doi: 10.3390/cells11091586. Cells. 2022. PMID: 35563892 Free PMC article.
-
Neonatal Hepatic Myeloid Progenitors Expand and Propagate Liver Injury in Mice.J Clin Med. 2023 Jan 1;12(1):337. doi: 10.3390/jcm12010337. J Clin Med. 2023. PMID: 36615137 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

