Feline Coronavirus and Alpha-Herpesvirus Infections: Innate Immune Response and Immune Escape Mechanisms
- PMID: 34944324
- PMCID: PMC8698202
- DOI: 10.3390/ani11123548
Feline Coronavirus and Alpha-Herpesvirus Infections: Innate Immune Response and Immune Escape Mechanisms
Abstract
Over time, feline viruses have acquired elaborateopportunistic properties, making their infections particularly difficult to prevent and treat. Feline coronavirus (FCoV) and feline herpesvirus-1 (FeHV-1), due to the involvement of host genetic factors and immune mechanisms in the development of the disease and more severe forms, are important examples of immune evasion of the host's innate immune response by feline viruses.It is widely accepted that the innate immune system, which providesan initial universal form of the mammalian host protection from infectious diseases without pre-exposure, plays an essential role in determining the outcome of viral infection.The main components of this immune systembranchare represented by the internal sensors of the host cells that are able to perceive the presence of viral component, including nucleic acids, to start and trigger the production of first type interferon and to activate the cytotoxicity by Natural Killercells, often exploited by viruses for immune evasion.In this brief review, we providea general overview of the principal tools of innate immunity, focusing on the immunologic escape implemented byFCoVand FeHV-1 duringinfection.
Keywords: FCoV; FIPV; FeHV-1; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
 and
and  , respectively.
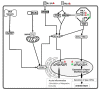
, respectively.  FeCoV infection induced higher gene expression level of TLRs 2, 4, 8 and 9, but not TLRs 3 and 7, suggesting either lack of an appropriate trigger, or virus inhibition of TLR trascripion. The synthesis of accessory proteins 7a and 3 by FeCoV are probably involved in the inhibition of type I IFN synthesis. The FIPV nsp5 produced an inhibition of IRF3 phosphorylation and suppression of type I IFN production.
FeCoV infection induced higher gene expression level of TLRs 2, 4, 8 and 9, but not TLRs 3 and 7, suggesting either lack of an appropriate trigger, or virus inhibition of TLR trascripion. The synthesis of accessory proteins 7a and 3 by FeCoV are probably involved in the inhibition of type I IFN synthesis. The FIPV nsp5 produced an inhibition of IRF3 phosphorylation and suppression of type I IFN production.  FeHV-1 infection induced an upregulation of TRL9 expression and a downregulation of TLR3. The FeHV-1 US3 protein competitively binds to IRF binding domain hindering dimerization of IRF3. TIR, Toll IL-1 receptor; TLR, Toll-like receptors; MyD88, Myeloid differentiation primary response 88; TRIF, TIR-domain-containing adapter-inducing interferon-β; NF-κB, Nuclear Factor kappa B; IRFs, Interferon Regulatory Factors; IFN, Interferon; IKK, Inhibitor-KbKinase; TBK1, TANK Binding Kinase 1; cGAMP, Cyclic guanosine monophosphate-adenosine monophosphate; STING, Stimulator of Interferon Genes; RLRs, RIG-I-like receptors; MAVs, Mitochondrial antiviral-signaling protein; nsp, nonstructural protein.
FeHV-1 infection induced an upregulation of TRL9 expression and a downregulation of TLR3. The FeHV-1 US3 protein competitively binds to IRF binding domain hindering dimerization of IRF3. TIR, Toll IL-1 receptor; TLR, Toll-like receptors; MyD88, Myeloid differentiation primary response 88; TRIF, TIR-domain-containing adapter-inducing interferon-β; NF-κB, Nuclear Factor kappa B; IRFs, Interferon Regulatory Factors; IFN, Interferon; IKK, Inhibitor-KbKinase; TBK1, TANK Binding Kinase 1; cGAMP, Cyclic guanosine monophosphate-adenosine monophosphate; STING, Stimulator of Interferon Genes; RLRs, RIG-I-like receptors; MAVs, Mitochondrial antiviral-signaling protein; nsp, nonstructural protein.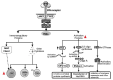
 .
.  FeCoV infection inhibited the type I IFN synthesis that results in an inhibition of the protein synthesis. Natural Killer (NK) cells are drastically depleted from the peripheral blood mesenteric lymph nodes and spleen in FIPV-infected cats. Moreover, NK showed less cytotoxic activity in FIP-infected cats. dsRNA, Double-stranded RNA; PKR, Double-stranded RNA-activated protein kinase, EIF2-a, Eukaryotic translation initiation factor 2A; RNAse-L, Ribonuclease L; MHC, Major Histocompatibility Complex; NK, Natural Killer; Naive T, Naïve T cells; CD8+, Cytotoxic T lymphocytes; JAK1, Janus Kinase 1; TYK2, Tyrosine kinase 2; STAT1, Signal Transducer and Activator of Transcription 1; STAT2, Signal Transducer and Activator of Transcription 2; Mx GTPases, Mx dynamin-like GTPases; ISGs, Interferon stimulated genes.
FeCoV infection inhibited the type I IFN synthesis that results in an inhibition of the protein synthesis. Natural Killer (NK) cells are drastically depleted from the peripheral blood mesenteric lymph nodes and spleen in FIPV-infected cats. Moreover, NK showed less cytotoxic activity in FIP-infected cats. dsRNA, Double-stranded RNA; PKR, Double-stranded RNA-activated protein kinase, EIF2-a, Eukaryotic translation initiation factor 2A; RNAse-L, Ribonuclease L; MHC, Major Histocompatibility Complex; NK, Natural Killer; Naive T, Naïve T cells; CD8+, Cytotoxic T lymphocytes; JAK1, Janus Kinase 1; TYK2, Tyrosine kinase 2; STAT1, Signal Transducer and Activator of Transcription 1; STAT2, Signal Transducer and Activator of Transcription 2; Mx GTPases, Mx dynamin-like GTPases; ISGs, Interferon stimulated genes.Similar articles
-
Innate Immune Evasion by Human Respiratory RNA Viruses.J Innate Immun. 2020;12(1):4-20. doi: 10.1159/000503030. Epub 2019 Oct 14. J Innate Immun. 2020. PMID: 31610541 Free PMC article. Review.
-
Expression of Toll-like receptors 3, 7, 9 and cytokines in feline infectious peritonitis virus-infected CRFK cells and feline peripheral monocytes.J Vet Sci. 2022 Mar;23(2):e27. doi: 10.4142/jvs.21225. J Vet Sci. 2022. PMID: 35363438 Free PMC article.
-
The Effect of Natural Feline Coronavirus Infection on the Host Immune Response: A Whole-Transcriptome Analysis of the Mesenteric Lymph Nodes in Cats with and without Feline Infectious Peritonitis.Pathogens. 2020 Jun 29;9(7):524. doi: 10.3390/pathogens9070524. Pathogens. 2020. PMID: 32610501 Free PMC article.
-
Establishment of Full-Length cDNA Clones and an Efficient Oral Infection Model for Feline Coronavirus in Cats.J Virol. 2021 Oct 13;95(21):e0074521. doi: 10.1128/JVI.00745-21. Epub 2021 Aug 18. J Virol. 2021. PMID: 34406859 Free PMC article.
-
Insights on the cGAS-STING Signaling Pathway During Herpesvirus Infections.Front Immunol. 2022 Jul 1;13:931885. doi: 10.3389/fimmu.2022.931885. eCollection 2022. Front Immunol. 2022. PMID: 35844623 Free PMC article. Review.
Cited by
-
The Enhancement of Immunity Gained from Feline Trivalent Vaccines in Mice Using Feline IL-15, IL-23 and Metabolic Regulatory Molecules.Biology (Basel). 2025 Jul 9;14(7):834. doi: 10.3390/biology14070834. Biology (Basel). 2025. PMID: 40723393 Free PMC article.
-
Cellular and humoral immune responses in cats vaccinated with feline herpesvirus 1 modified live virus vaccine.Front Vet Sci. 2025 Jan 15;11:1516850. doi: 10.3389/fvets.2024.1516850. eCollection 2024. Front Vet Sci. 2025. PMID: 39881722 Free PMC article.
-
A Combined Method Based on the FIPV N Monoclonal Antibody Immunofluorescence Assay and RT-nPCR Method for the Rapid Diagnosis of FIP-Suspected Ascites.Transbound Emerg Dis. 2023 Mar 28;2023:8429106. doi: 10.1155/2023/8429106. eCollection 2023. Transbound Emerg Dis. 2023. PMID: 40303664 Free PMC article.
-
In Vitro Evaluation of Aryl Hydrocarbon Receptor Involvement in Feline Coronavirus Infection.Viruses. 2025 Feb 6;17(2):227. doi: 10.3390/v17020227. Viruses. 2025. PMID: 40006982 Free PMC article.
-
Advances in the immunoescape mechanisms exploited by alphaherpesviruses.Front Microbiol. 2024 Jun 19;15:1392814. doi: 10.3389/fmicb.2024.1392814. eCollection 2024. Front Microbiol. 2024. PMID: 38962133 Free PMC article. Review.
References
-
- Robert-Tissot C., Ruegger V.L., Cattori V., Meli M.L., Riond B., Moore P.F., Engels M., Franchini M., Hofmann-Lehmann R., Lutz H. Stimulation with a class A CpG oligonucleotide enhances resistance to infection with feline viruses from five different families. Vet. Res. 2012;43:60. doi: 10.1186/1297-9716-43-60. - DOI - PMC - PubMed
-
- Malbon A.J., Meli M.L., Barker E.N., Davidson A.D., Tasker S., Kipar A. Inflammatory Mediators in the Mesenteric Lymph Nodes, Site of a Possible Intermediate Phase in the Immune Response to Feline Coronavirus and the Pathogenesis of Feline Infectious Peritonitis? J. Comp. Pathol. 2019;166:69–86. doi: 10.1016/j.jcpa.2018.11.001. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

