Utero-Placental Immune Milieu during Normal and Aglepristone-Induced Parturition in the Dog
- PMID: 34944375
- PMCID: PMC8697996
- DOI: 10.3390/ani11123598
Utero-Placental Immune Milieu during Normal and Aglepristone-Induced Parturition in the Dog
Abstract
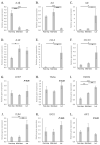
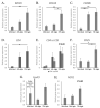
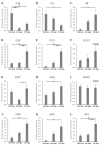
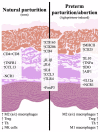
Maternal immunotolerance is required for the maintenance of pregnancy, in sharp contrast with the uterine pro-inflammatory activity observed during parturition in several species. Correspondingly, in the dog, increased immune signaling at term has been suggested, but a deeper understanding of the uterine immune milieu is still missing. Thus, the availability of 30 immune-related factors was assessed in utero-placental samples collected during post-implantation (days 18-25 of pregnancy) and mid-gestation (days 35-40) stages, and at the time of prepartum luteolysis. Gene expression and/or protein localization studies were employed. Samples collected from antigestagen (aglepristone)-treated dogs were further analyzed. Progression of pregnancy was associated with the downregulation of IL1β and upregulation of IL10 (p < 0.05) at mid-gestation. When compared with mid-gestation, a higher availability of several factors was observed at term (e.g., CD206, CD4, TLR4). However, in contrast with natural parturition, MHCII, CD25, CCR7, TNFα, IDO1 and AIF1 were upregulated after aglepristone treatment (p < 0.05), but not TNFR1 or CCL13 (p > 0.05). Altogether, these results show an increased immune activity during canine parturition, involving, i.a., M2 macrophages, Treg and Th cells, with strong support for progesterone-mediated immunomodulation. Furthermore, differences between term and induced parturition/abortion could relate to differences in placental maturation towards parturition and/or functional traits of antigestagens.
Keywords: aglepristone; dog (Canis lupus familiaris); immune system; parturition; utero-placental.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

