Regulation of the Immune Checkpoint Indoleamine 2,3-Dioxygenase Expression by Epstein-Barr Virus
- PMID: 34944437
- PMCID: PMC8699098
- DOI: 10.3390/biom11121792
Regulation of the Immune Checkpoint Indoleamine 2,3-Dioxygenase Expression by Epstein-Barr Virus
Abstract
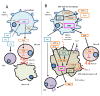
Epstein-Barr virus (EBV) is an oncovirus ubiquitously distributed and associated with different types of cancer. The reason why only a group of infected people develop cancer is still unknown. EBV-associated cancers represent about 1.8% of all cancer deaths worldwide, with more than 150,000 new cases of cancer being reported annually. Since EBV-associated cancers are described as more aggressive and more resistant to the usual treatment compared to EBV-negative ones, the recent introduction of monoclonal antibodies (mAbs) targeting immune checkpoints (ICs) in the treatment of cancer patients represents a possible therapy for EBV-associated diseases. However, the current mAb therapies available still need improvement, since a group of patients do not respond well to treatment. Therefore, the main objective of this review is to summarize the progress made regarding the contribution of EBV infection to the expression of the IC indoleamine 2,3-dioxygenase (IDO) thus far. This IC has the potential to be used as a target in new immune therapies, such as mAbs. We hope that this work helps the development of future immunotherapies, improving the prognosis of EBV-associated cancer patients.
Keywords: Epstein–Barr virus; immune checkpoints; indoleamine 2,3-dioxygenase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mentzer A.J., Brenner N., Allen N., Littlejohns T.J., Chong A.Y., Cortes A., Almond R., Hill M., Sheard S., McVean G., et al. Identification of host-pathogen-disease relationships using a scalable Multiplex Serology platform in UK Biobank. medRxiv. 2019:19004960. doi: 10.1101/19004960. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

