Oligomeric States and Hydrodynamic Properties of Lysyl Oxidase-Like 2
- PMID: 34944490
- PMCID: PMC8699698
- DOI: 10.3390/biom11121846
Oligomeric States and Hydrodynamic Properties of Lysyl Oxidase-Like 2
Abstract
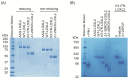
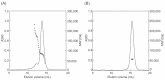
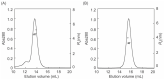
Lysyl oxidase-like 2 (LOXL2) has emerged as a promising therapeutic target against metastatic/invasive tumors and organ and tissue fibrosis. LOXL2 catalyzes the oxidative deamination of lysine and hydroxylysine residues in extracellular matrix (ECM) proteins to promote crosslinking of these proteins, and thereby plays a major role in ECM remodeling. LOXL2 secretes as 100-kDa full-length protein (fl-LOXL2) and then undergoes proteolytic cleavage of the first two scavenger receptor cysteine-rich (SRCR) domains to yield 60-kDa protein (Δ1-2SRCR-LOXL2). This processing does not affect the amine oxidase activity of LOXL2 in vitro. However, the physiological importance of this cleavage still remains elusive. In this study, we focused on characterization of biophysical properties of fl- and Δ1-2SRCR-LOXL2s (e.g., oligomeric states, molecular weights, and hydrodynamic radii in solution) to gain insight into the structural role of the first two SRCR domains. Our study reveals that fl-LOXL2 exists predominantly as monomer but also dimer to the lesser extent when its concentration is <~1 mM. The hydrodynamic radius (Rh) determined by multi-angle light scattering coupled with size exclusion chromatography (SEC-MALS) indicates that fl-LOXL2 is a moderately asymmetric protein. In contrast, Δ1-2SRCR-LOXL2 exists solely as monomer and its Rh is in good agreement with the predicted value. The Rh values calculated from a 3D modeled structure of fl-LOXL2 and the crystal structure of the precursor Δ1-2SRCR-LOXL2 are within a reasonable margin of error of the values determined by SEC-MALS for fl- and Δ1-2SRCR-LOXL2s in mature forms in this study. Based on superimposition of the 3D model and the crystal structure of Δ1-2SRCR-LOXL2 (PDB:5ZE3), we propose a configuration of fl-LOXL2 that explains the difference observed in Rh between fl- and Δ1-2SRCR-LOXL2s in solution.
Keywords: analytical ultracentrifugation; extracellular matrix; hydrodynamic radius; isoelectric points; lysyl oxidase-like 2; scavenger receptor cysteine-rich.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



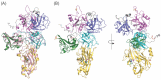
References
-
- Csiszar K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog. Nucleic Acid Res. Mol. Biol. 2001;70:1–32. - PubMed
-
- Schmelzer C.E.H., Heinz A., Troilo H., Lockhart-Cairns M.P., Jowitt T.A., Marchand M.F., Bidault L., Bignon M., Hedtke T., Barret A., et al. Lysyl oxidase-like 2 (LOXL2)-mediated cross-linking of tropoelastin. FASEB J. 2019;33:5468–5481. doi: 10.1096/fj.201801860RR. fj201801860RR. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

